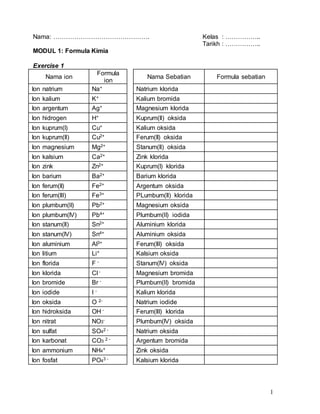
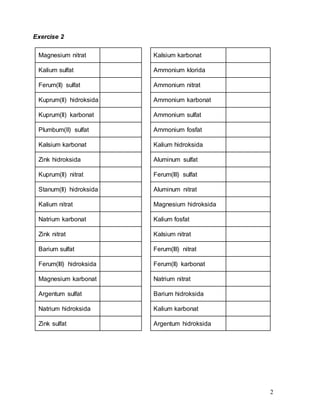
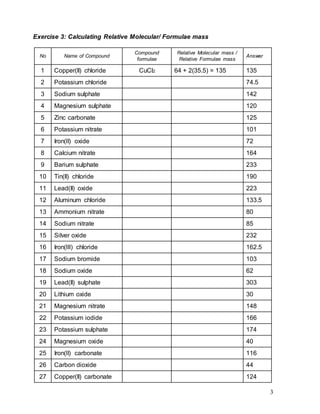
This document contains information about chemical formulas and relative molecular/formula masses. It includes a list of common ions and their formulas. There are also exercises providing the chemical formulas of compounds and calculating their relative molecular/formula masses. Some example calculations shown include copper(II) chloride with a relative formula mass of 135 and potassium chloride with a relative formula mass of 74.5. The document appears to be teaching materials for a chemistry module covering chemical formulas and relative molecular/formula mass calculations.