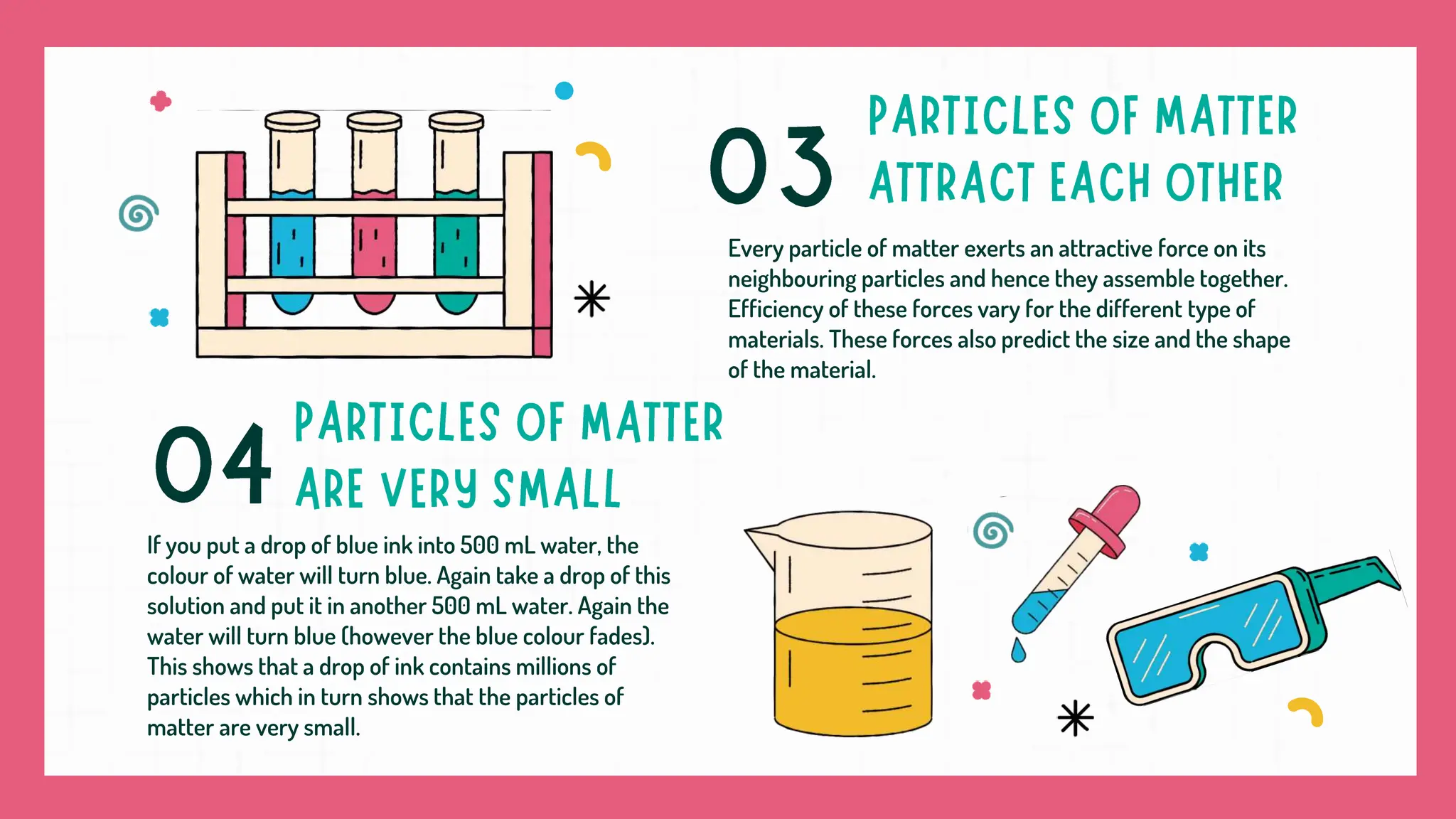
Matter is composed of particles that determine its nature and state, which exist as solids, liquids, or gases based on particle arrangement and movement. Changes in temperature and pressure can convert matter between states, including processes like melting, boiling, and sublimation. Evaporation occurs at temperatures below boiling point and is influenced by factors such as surface area, temperature, humidity, and wind speed.