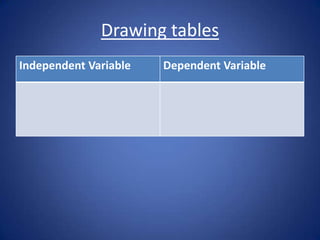
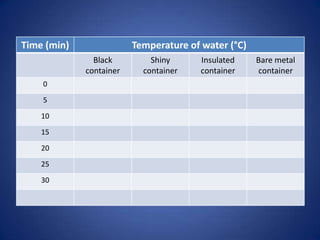
This document discusses heating and cooling through conduction, convection, and radiation. It explains that:
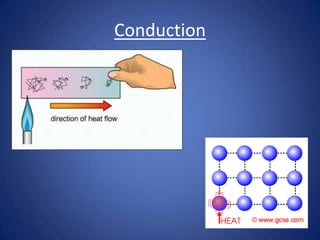
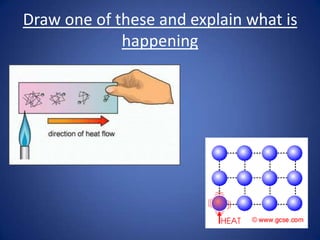
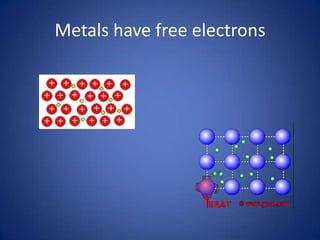
- Conduction involves the transfer of kinetic energy between particles in solids.
- Convection occurs in liquids and gases and involves the movement of particles with higher kinetic energy from hot to cold regions.
- Radiation transfers heat without particles and travels as waves, including how the sun's heat reaches Earth.
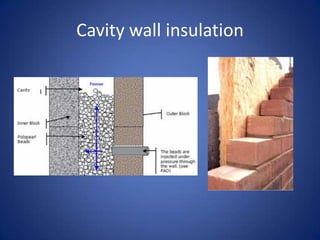
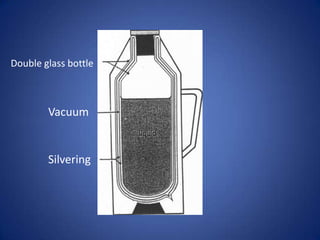
It then discusses methods of insulation, including cavity wall insulation and double glazing, and defines U-values as a measure of heat loss through materials.