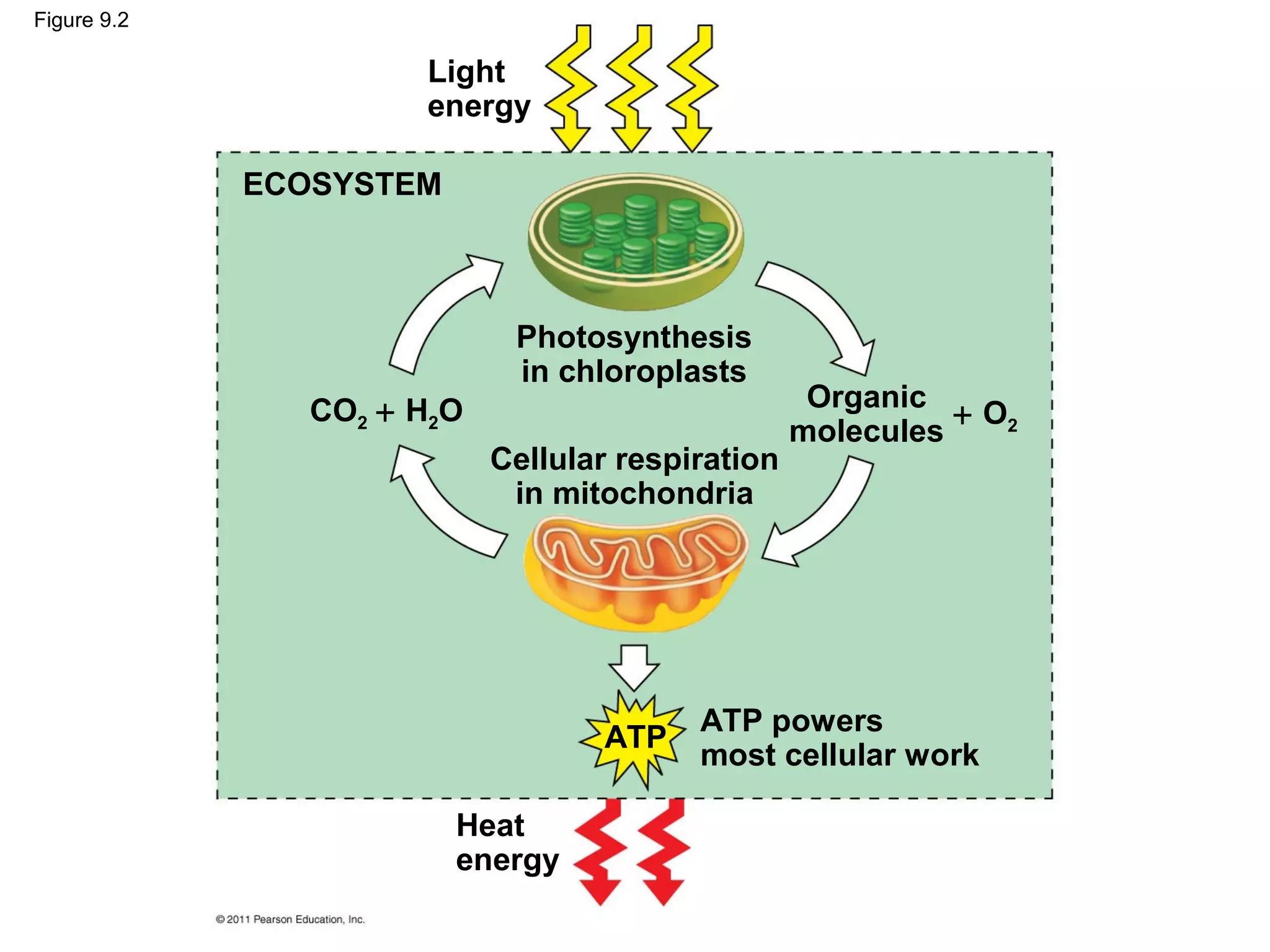
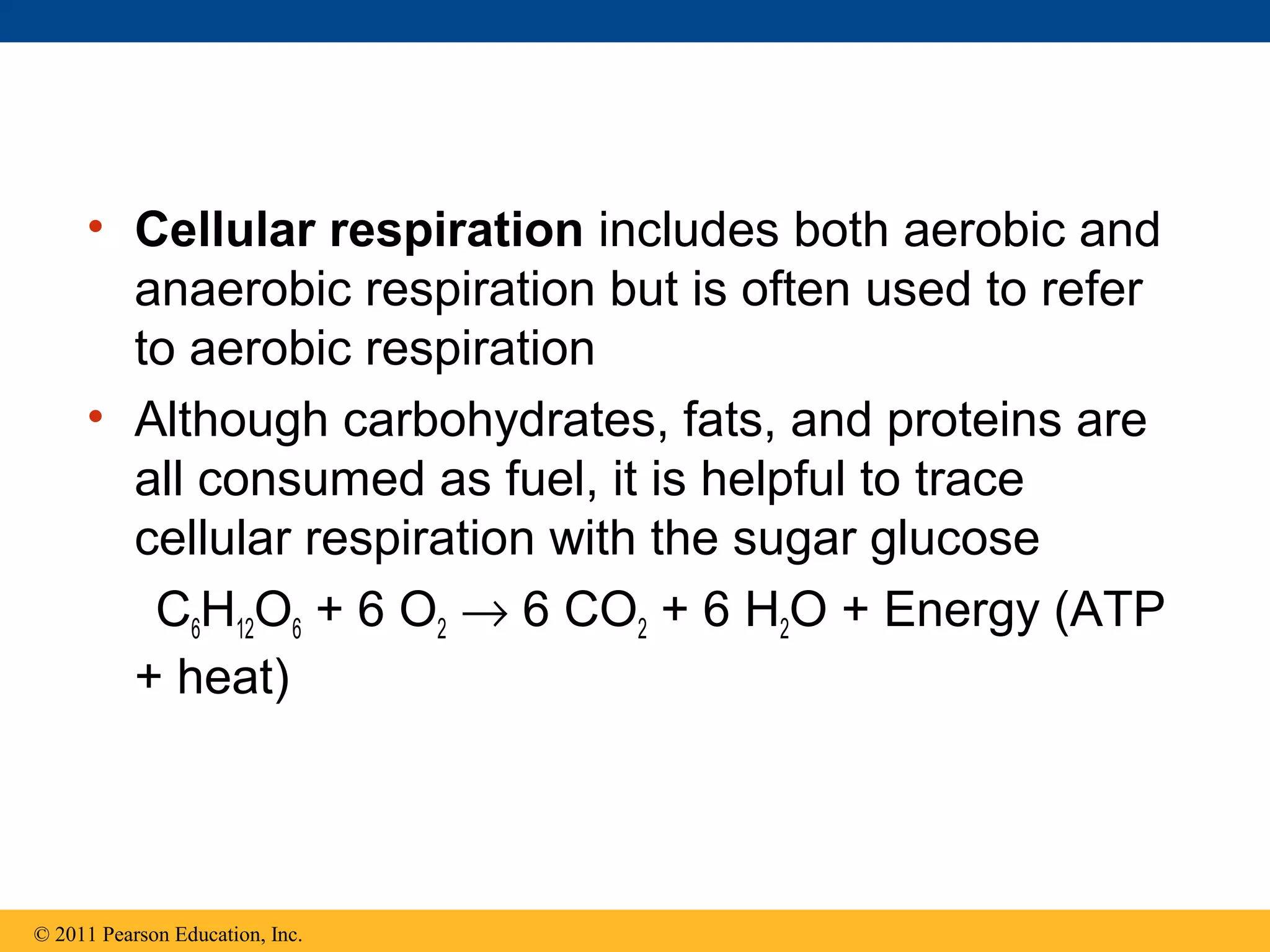
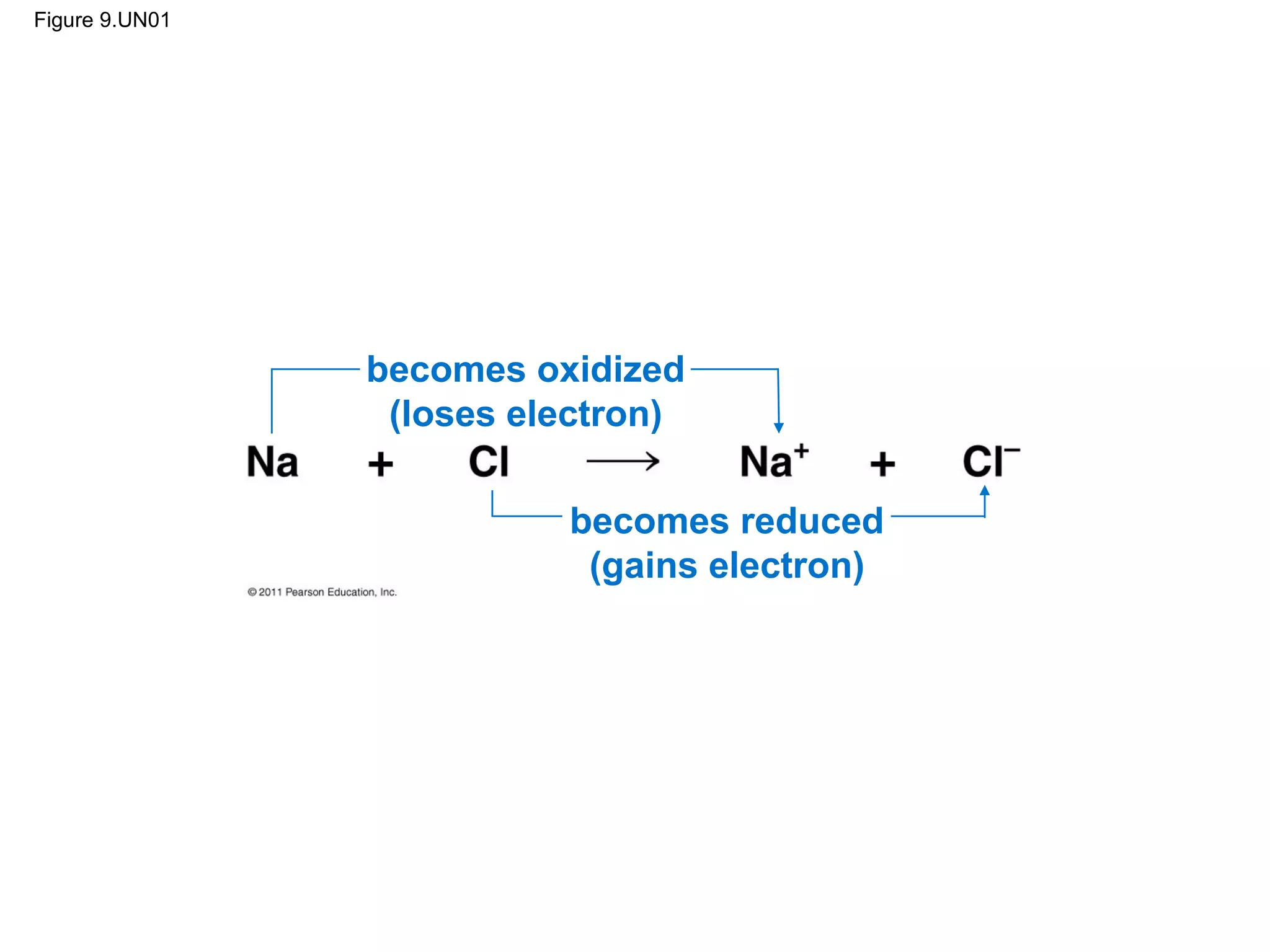
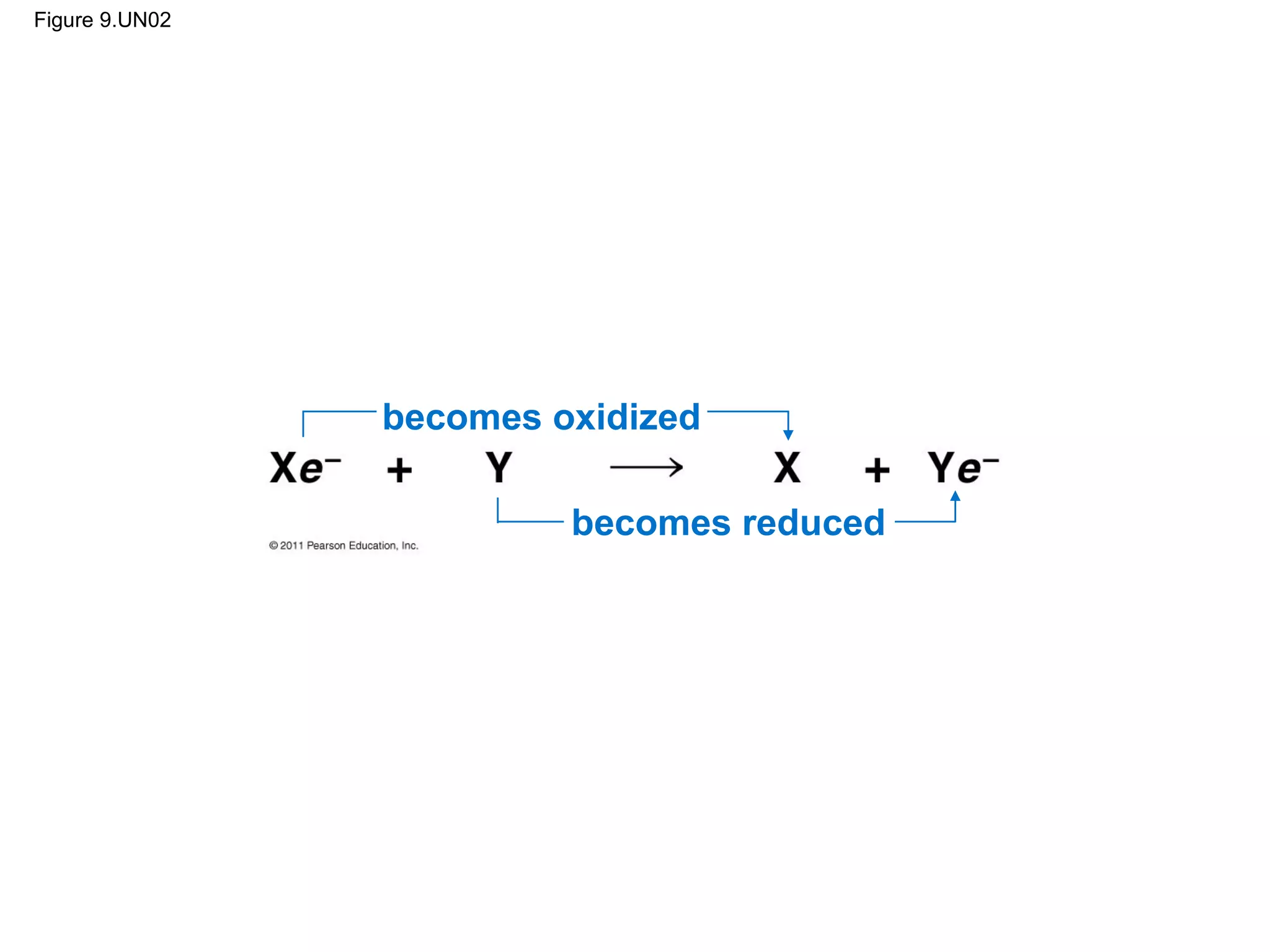
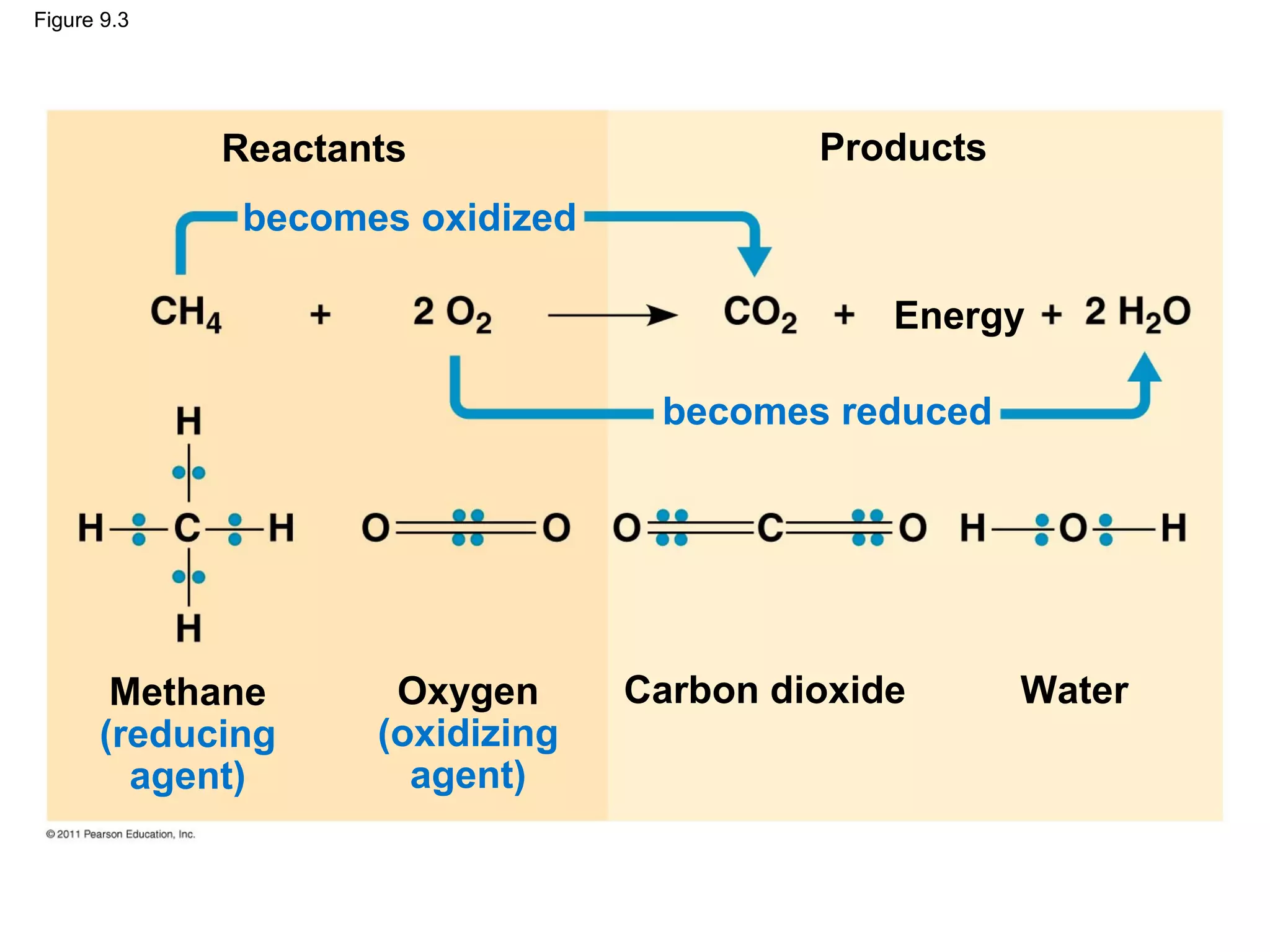
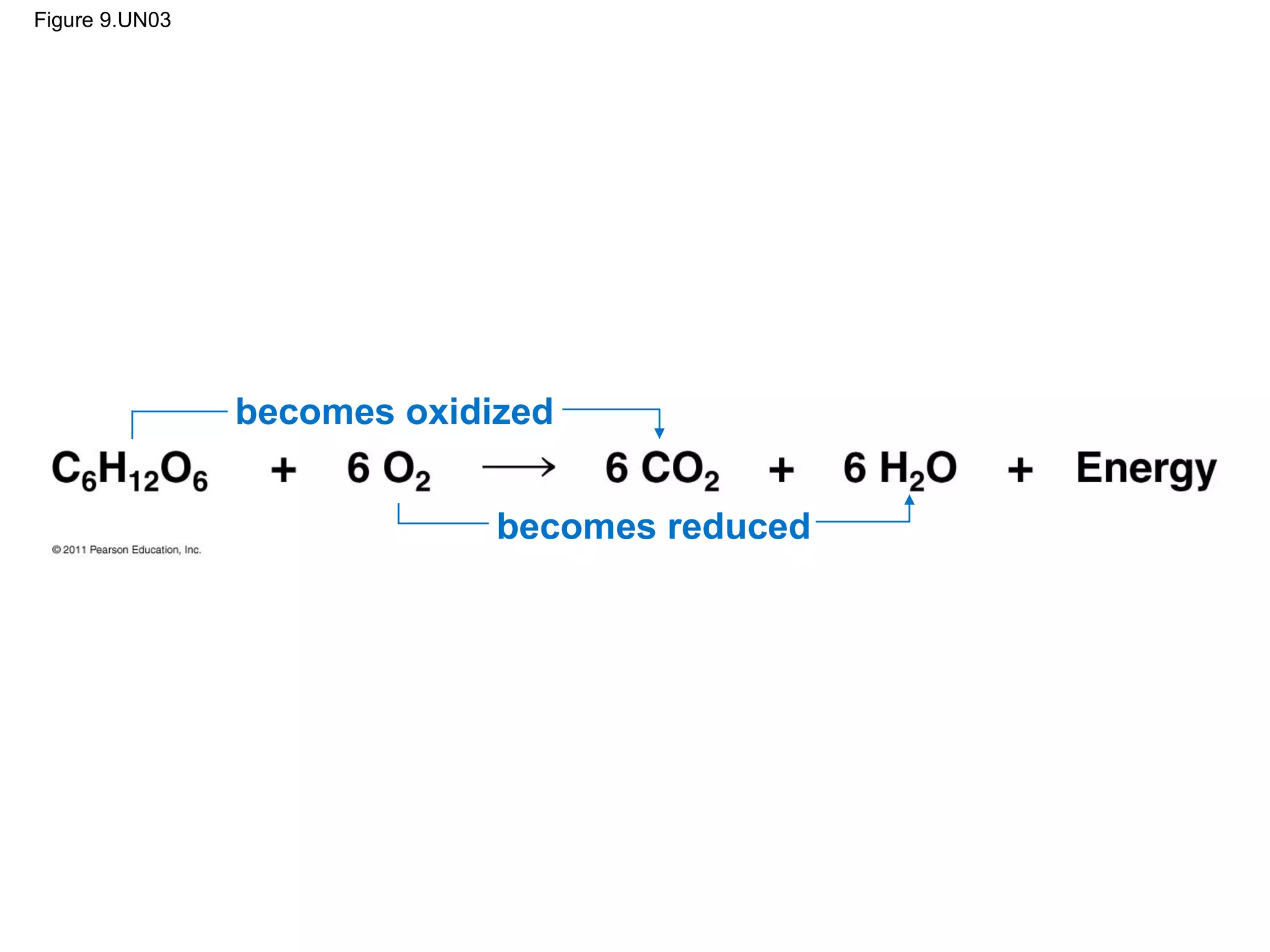
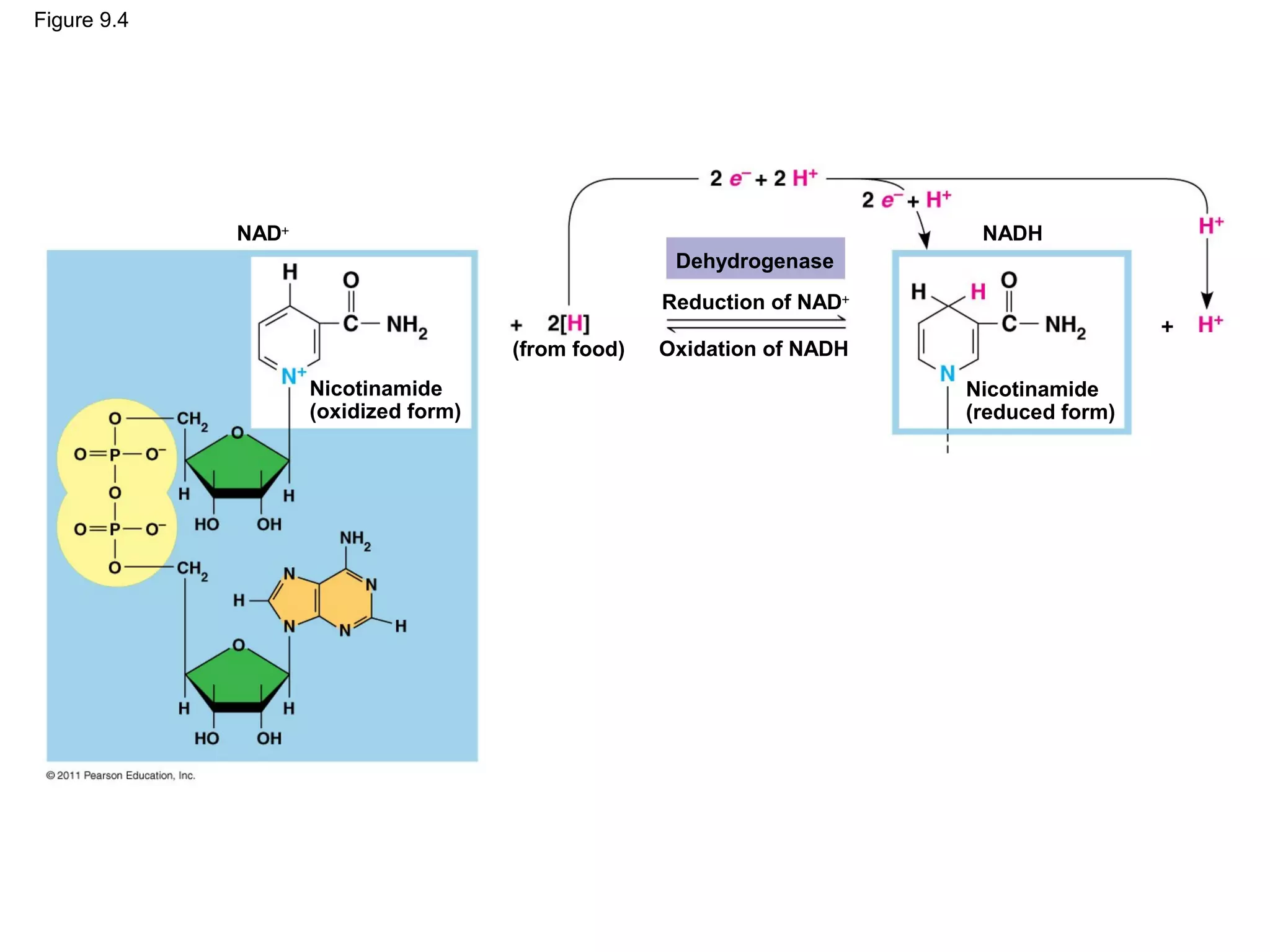
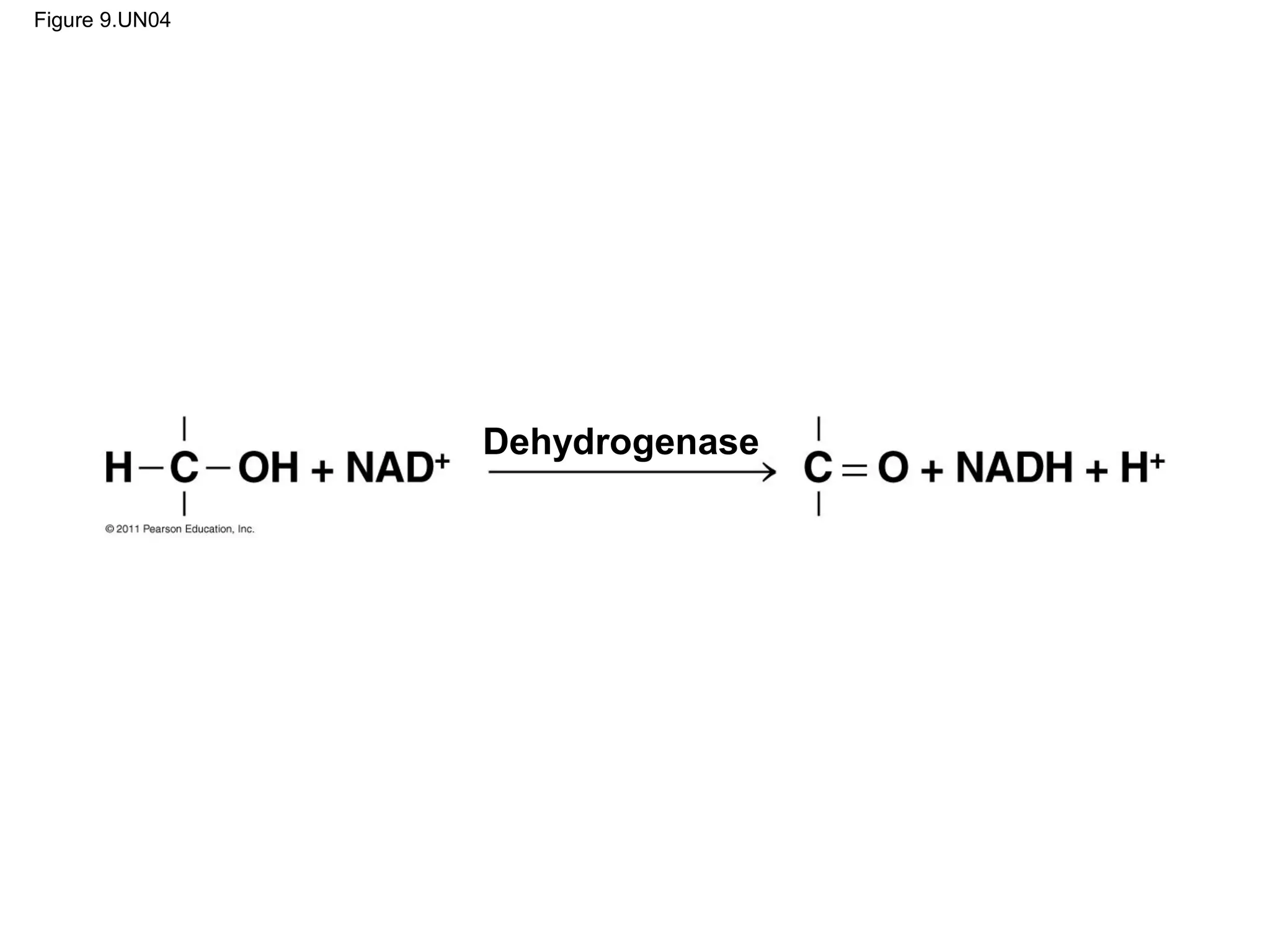
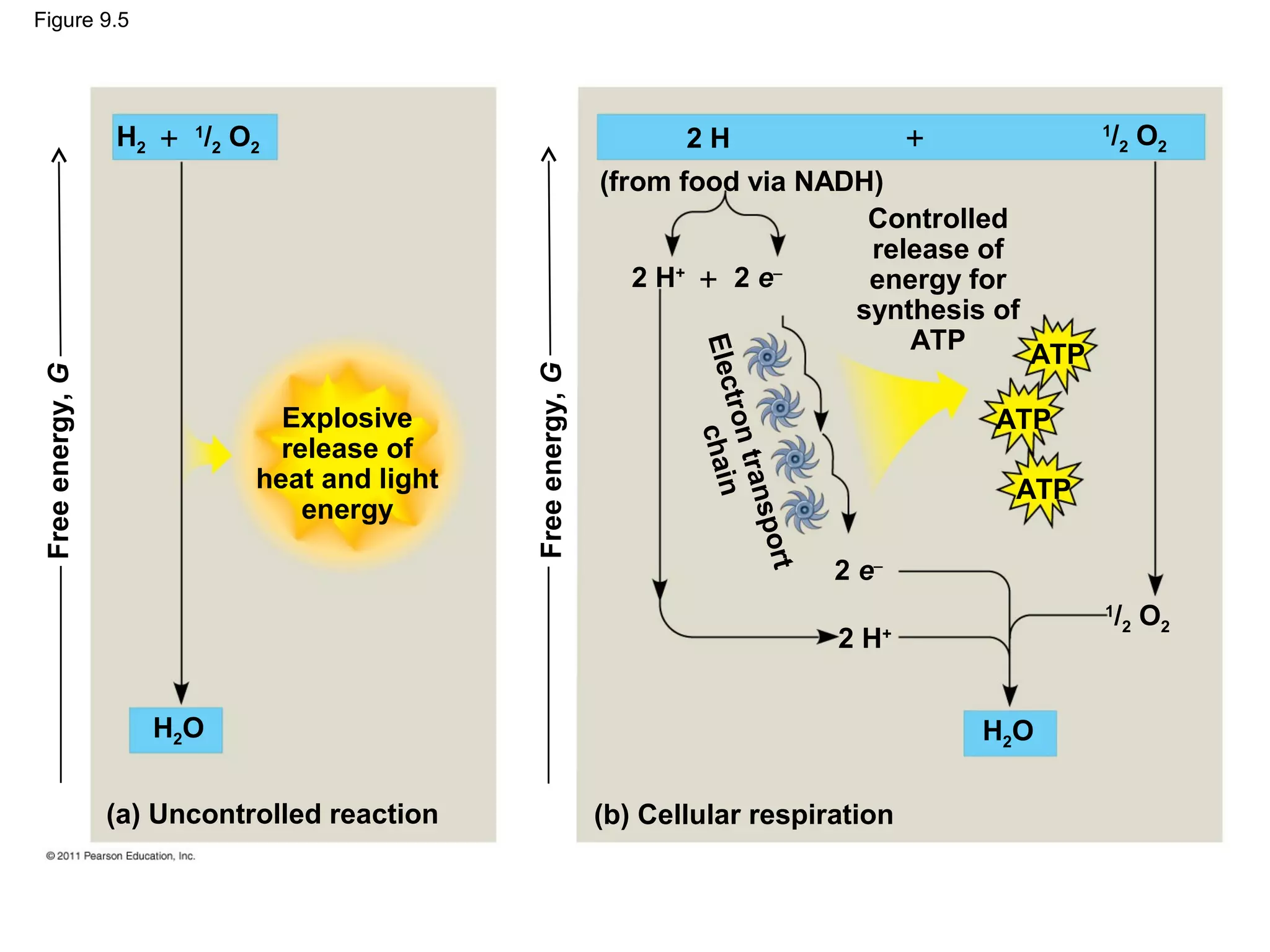
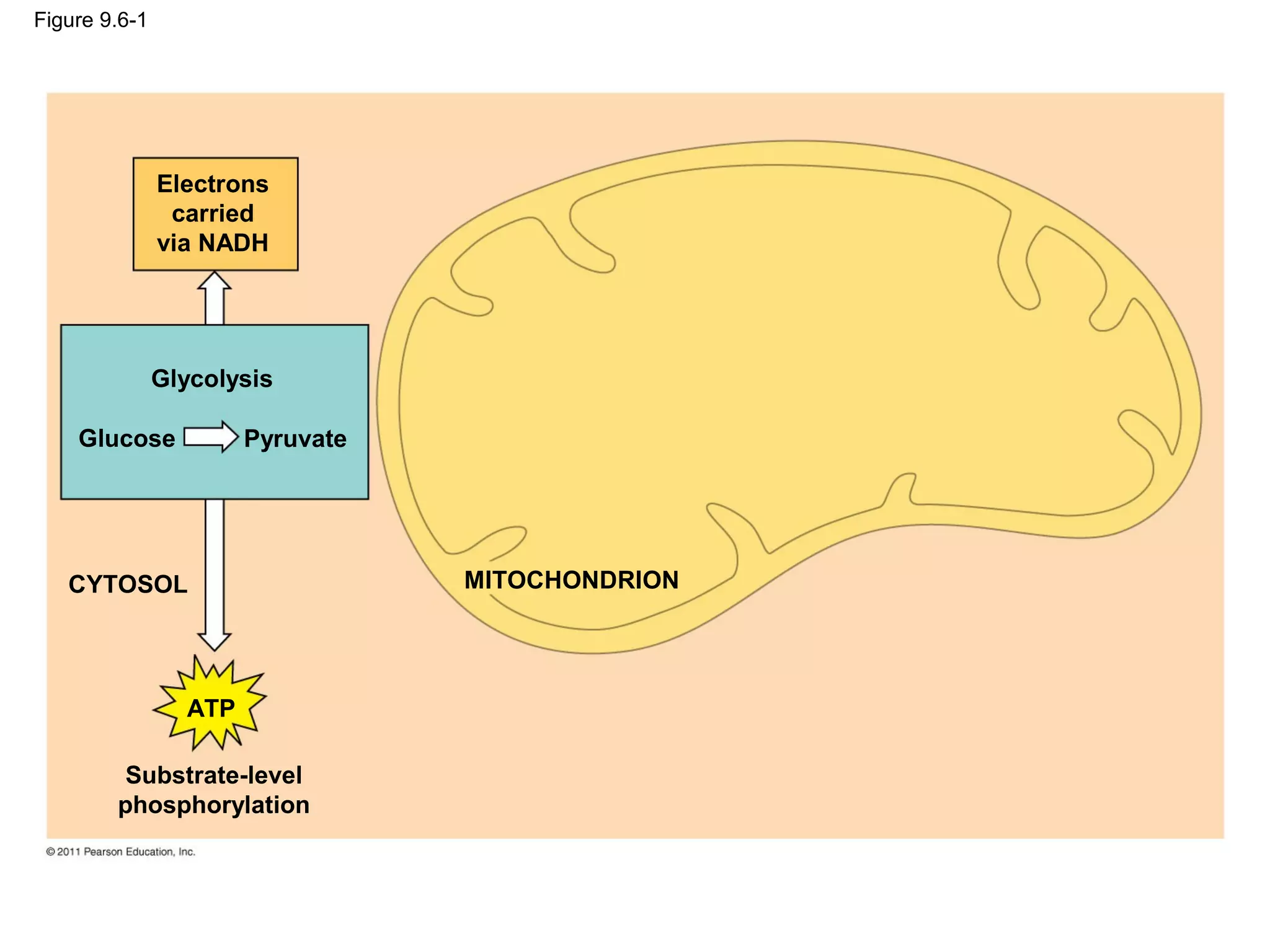
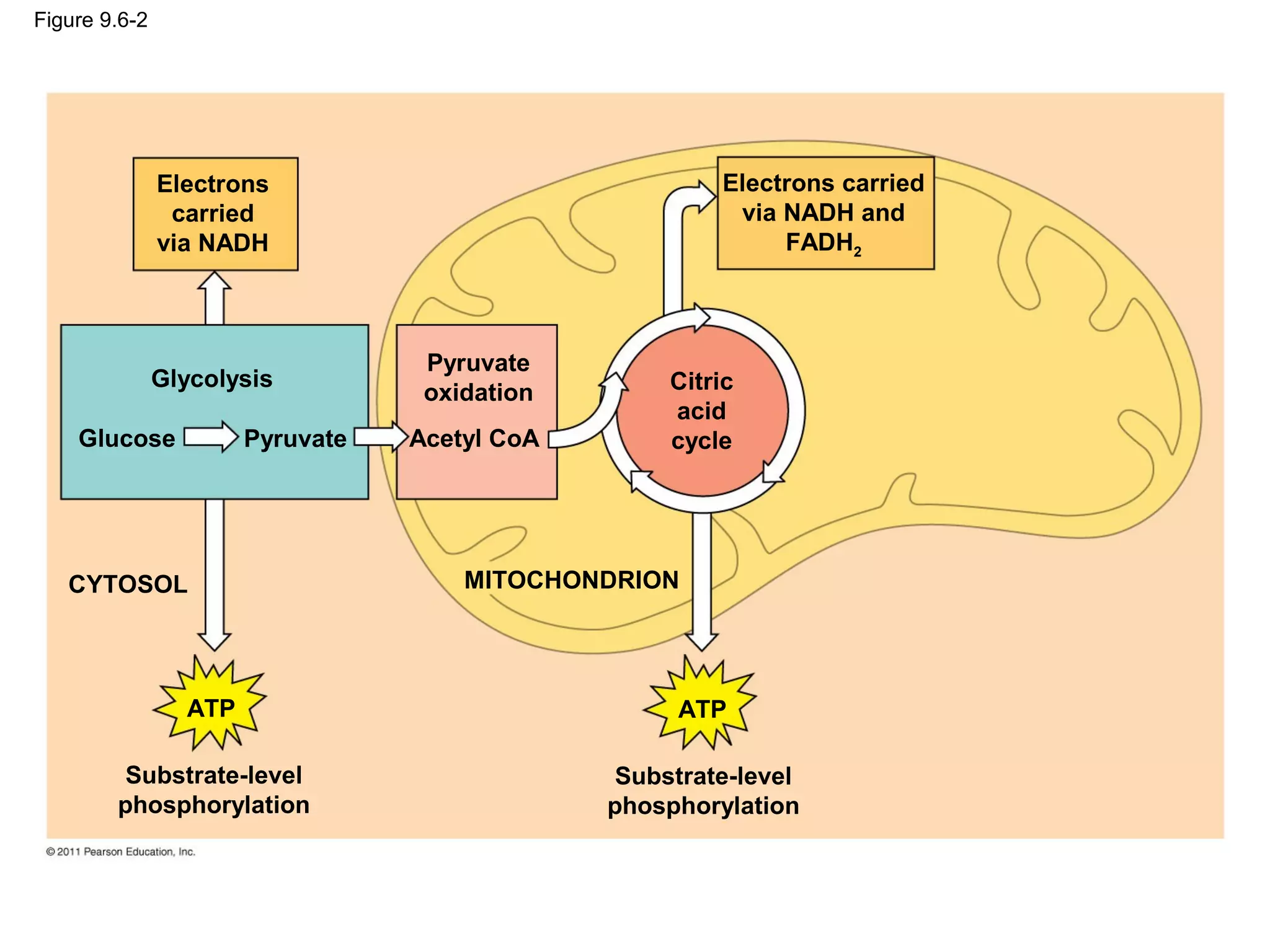
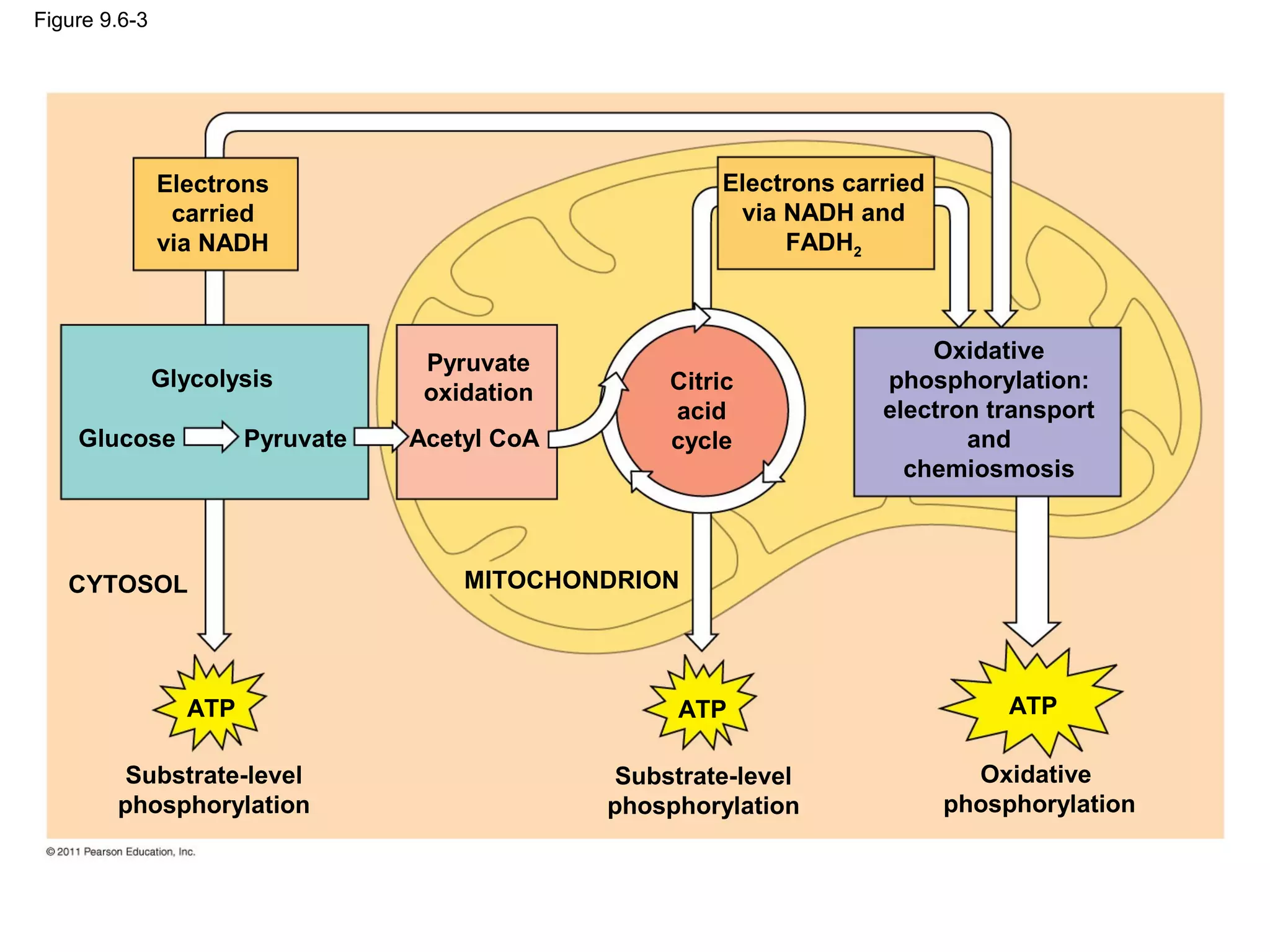
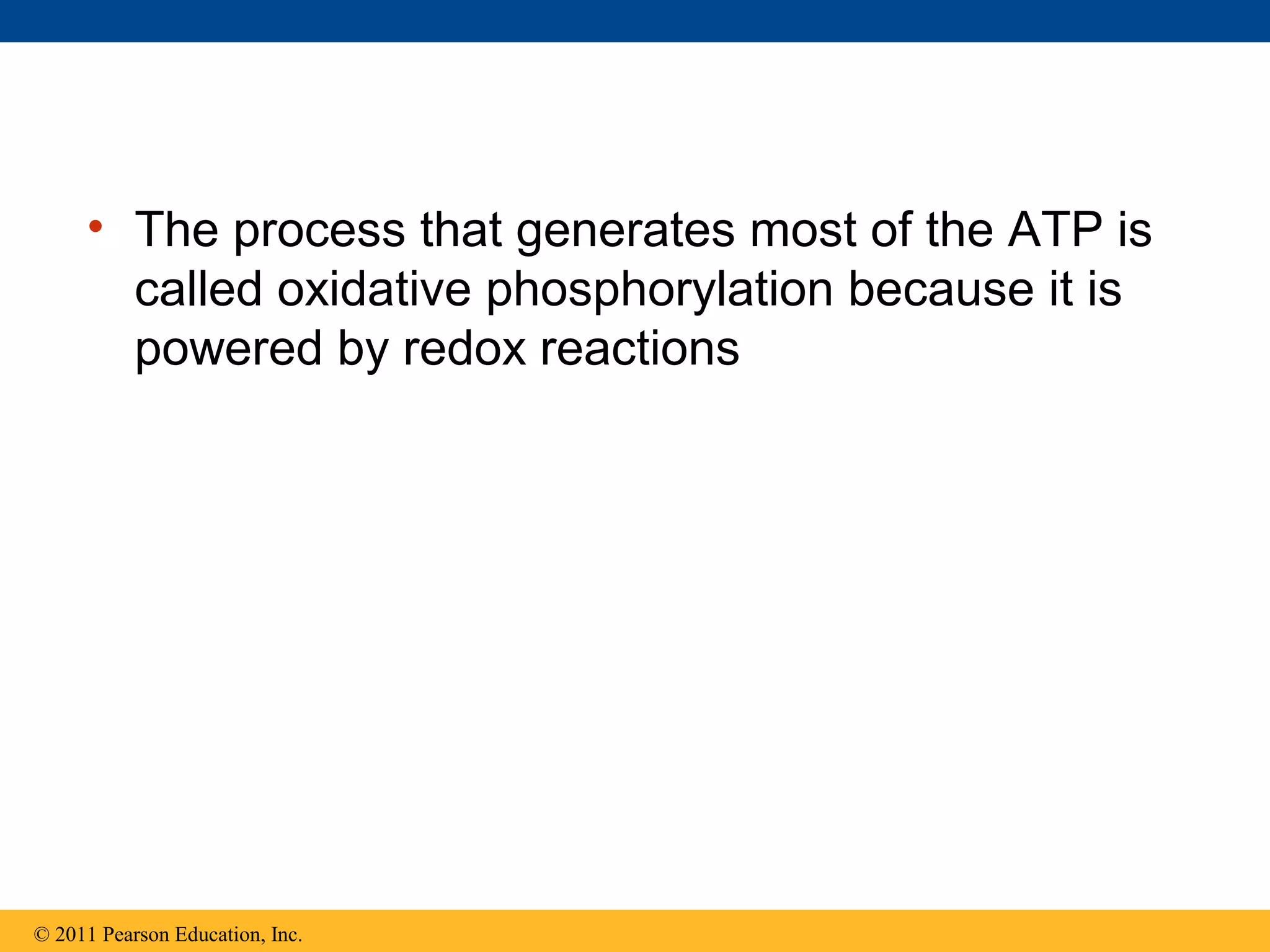
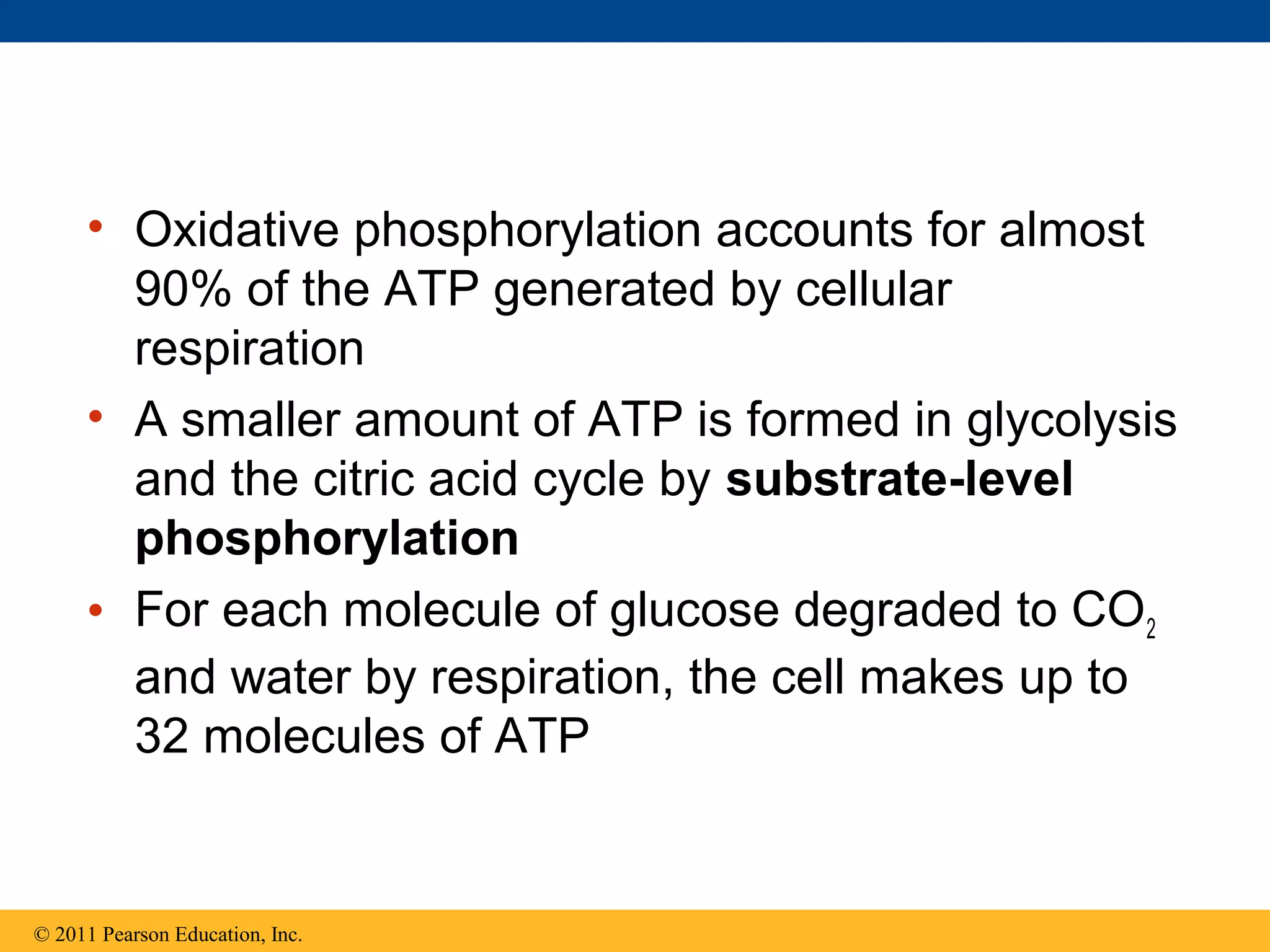
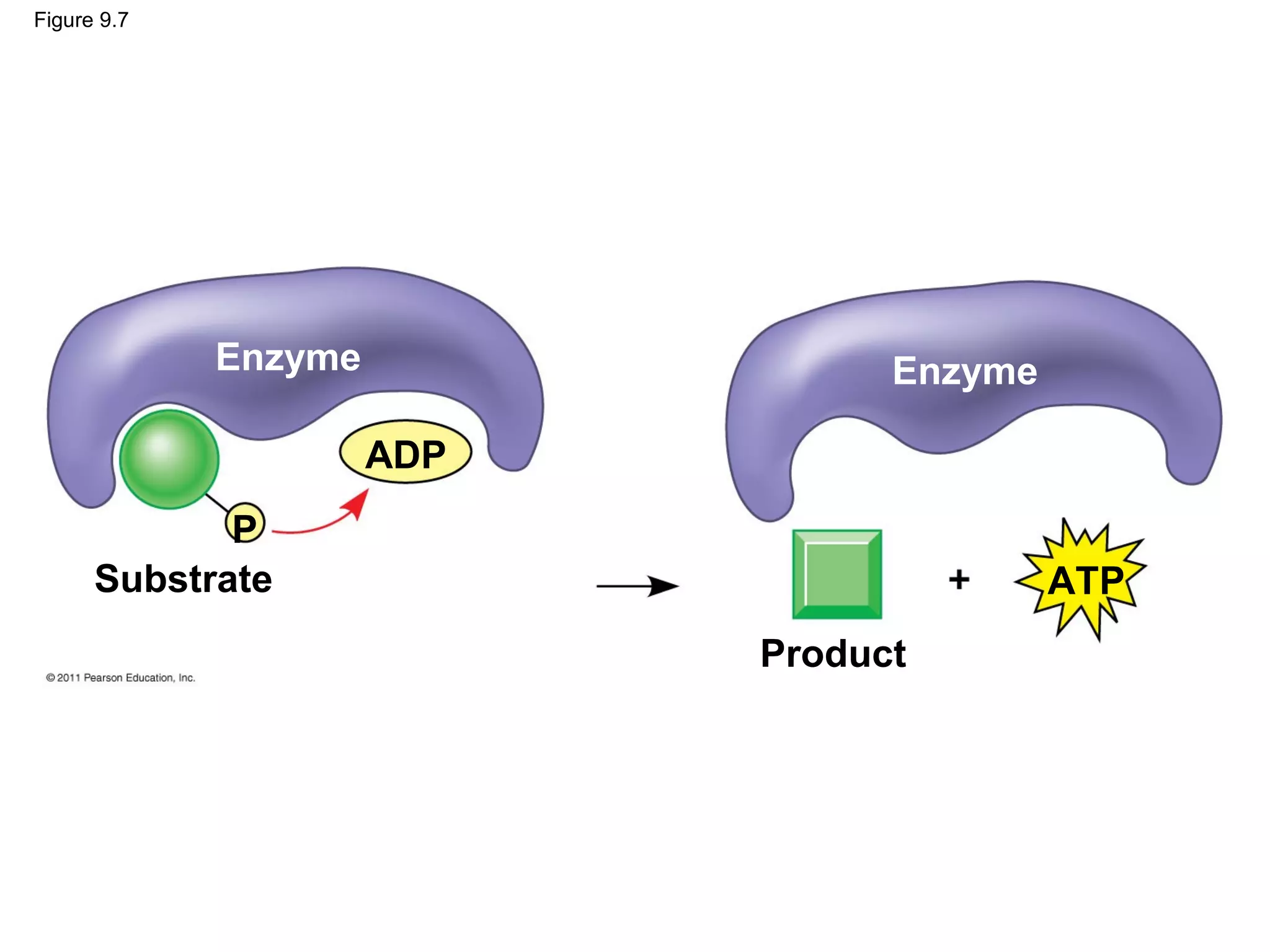
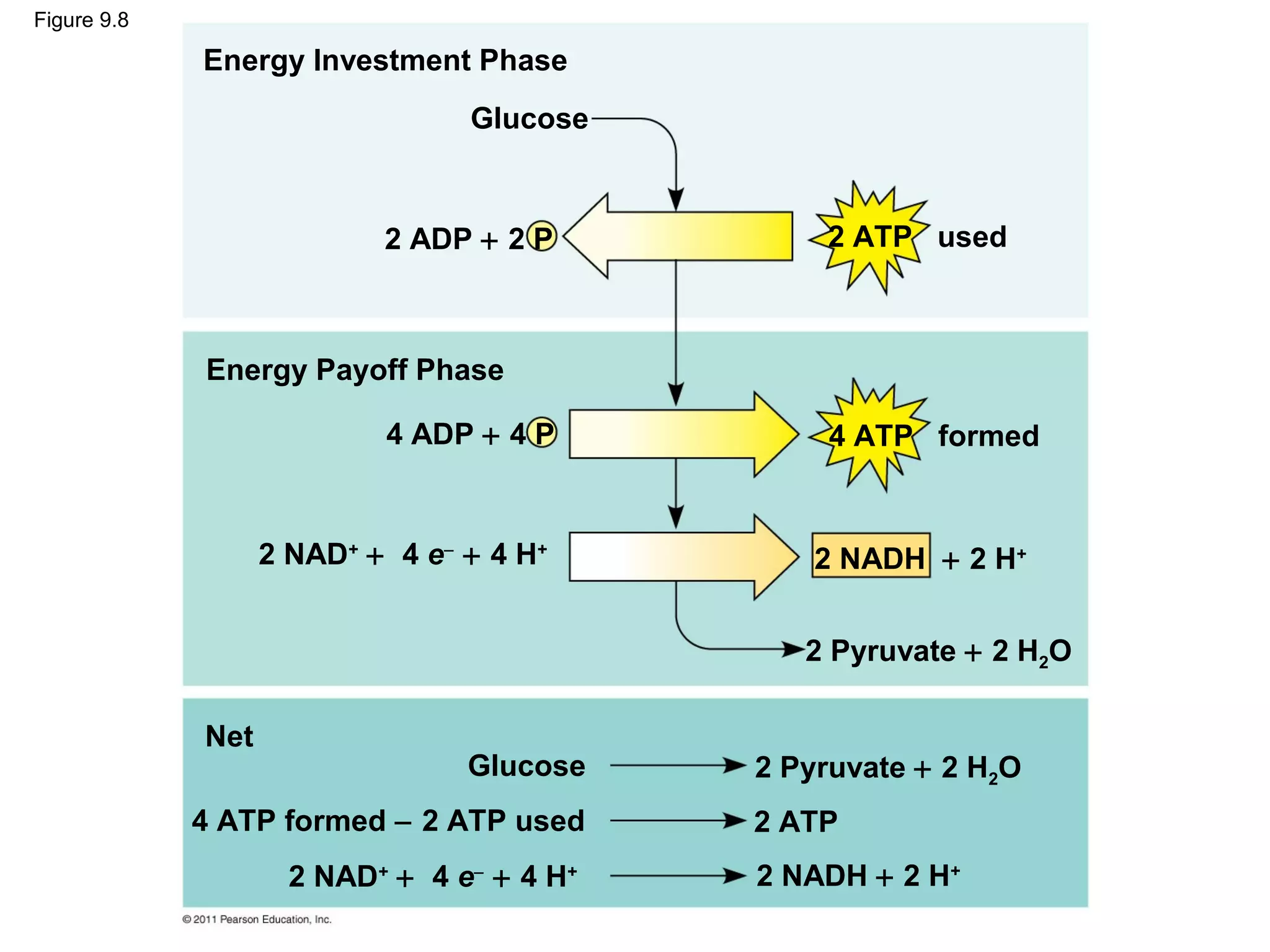
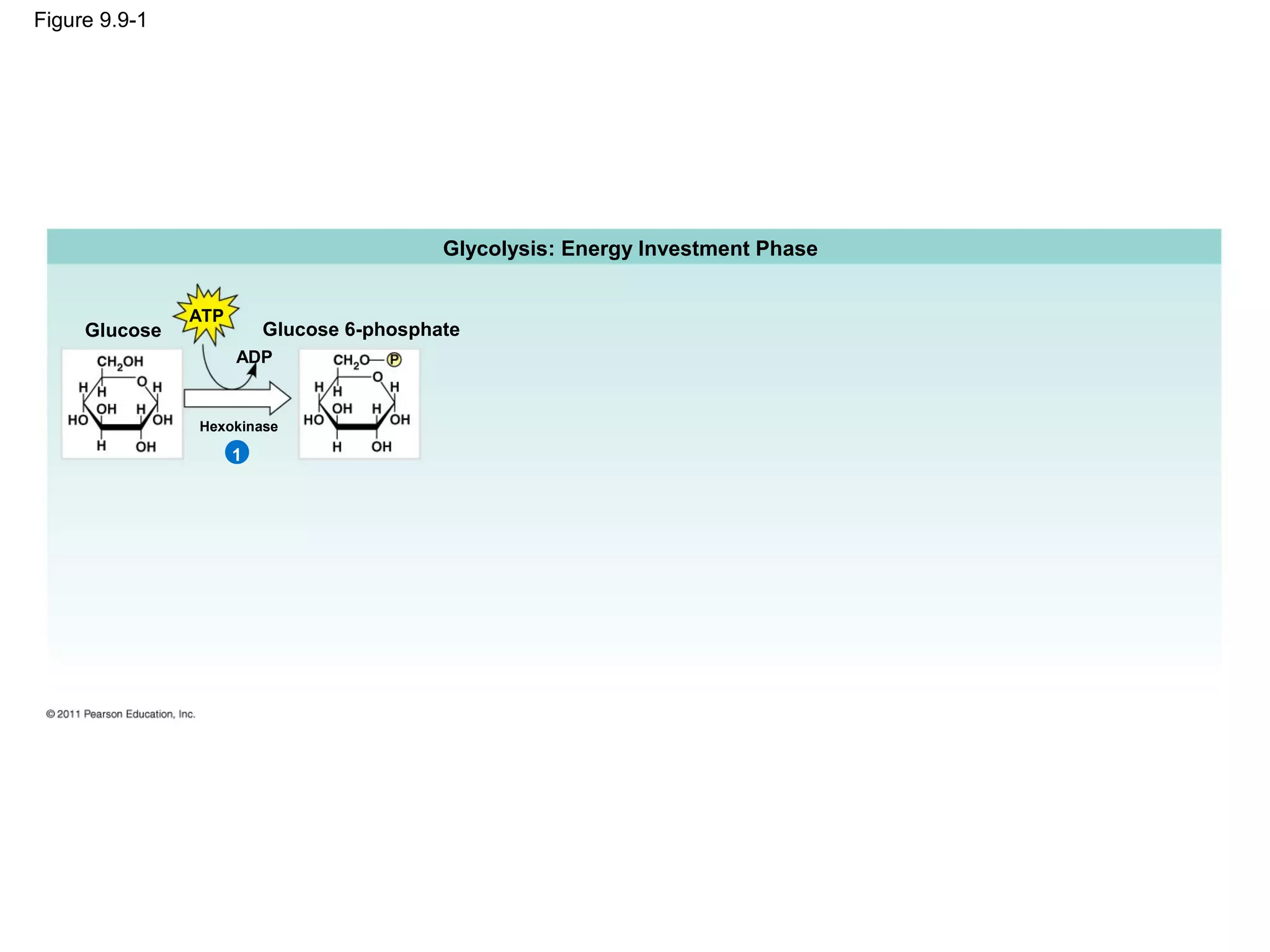
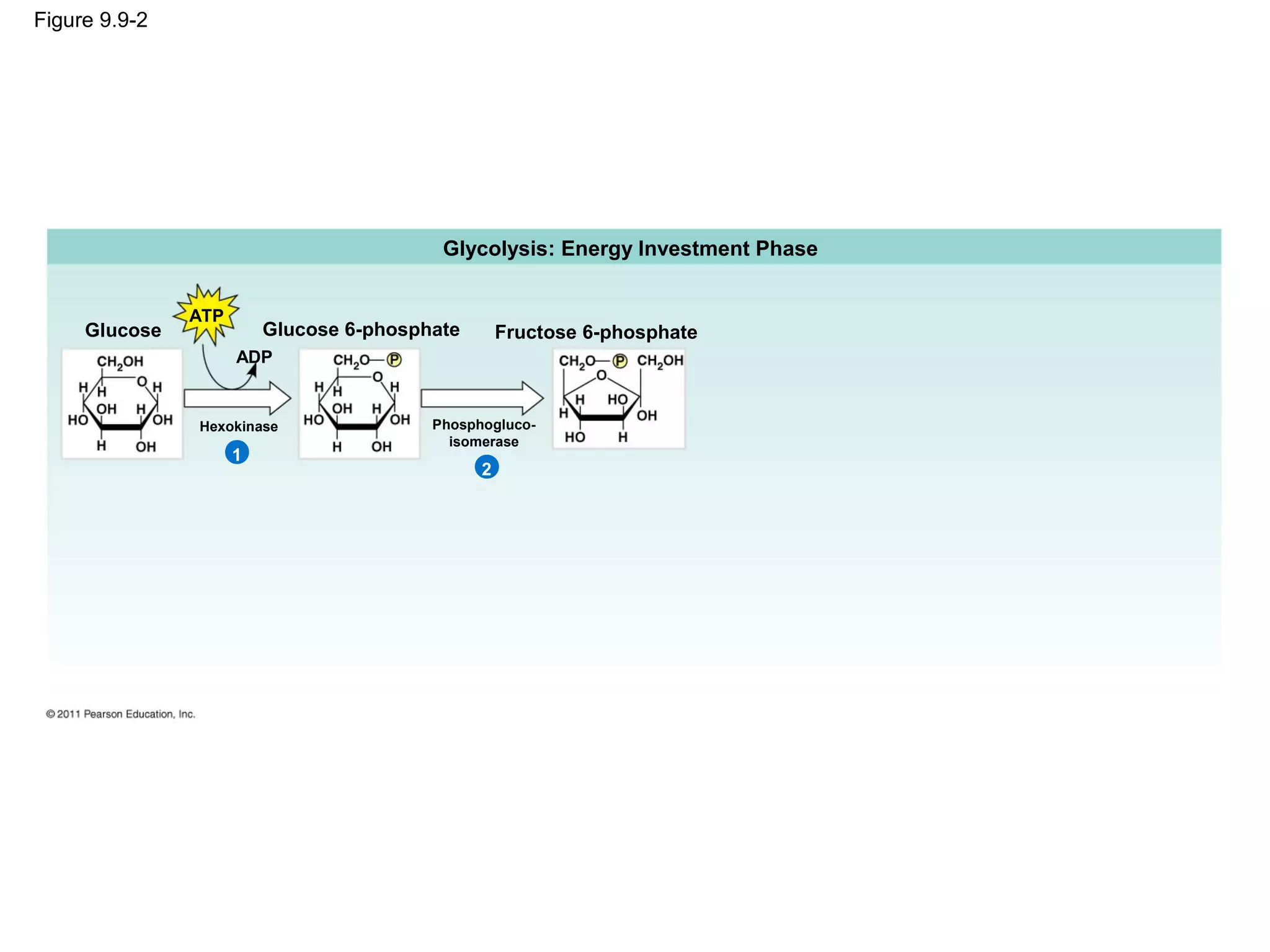
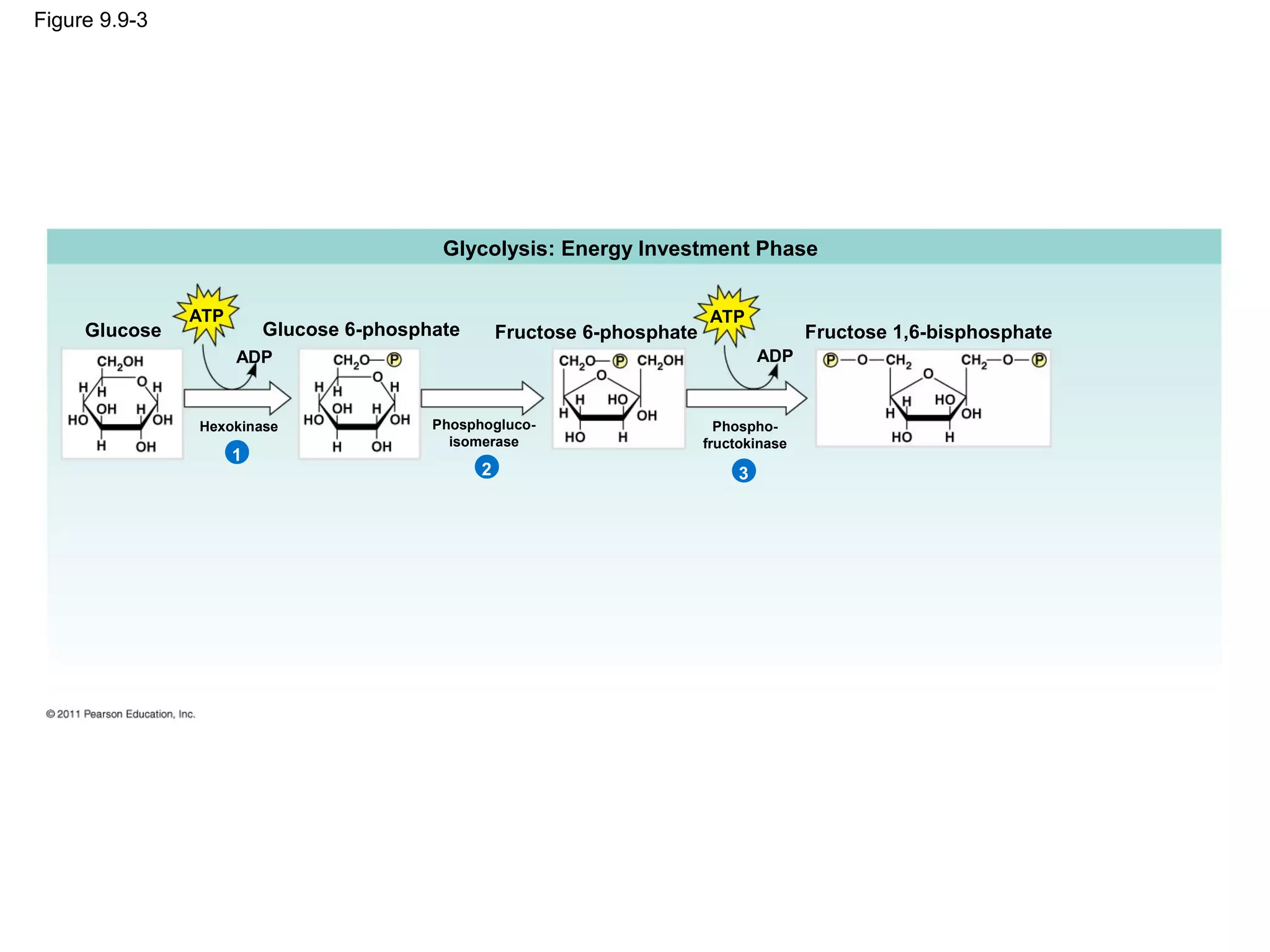
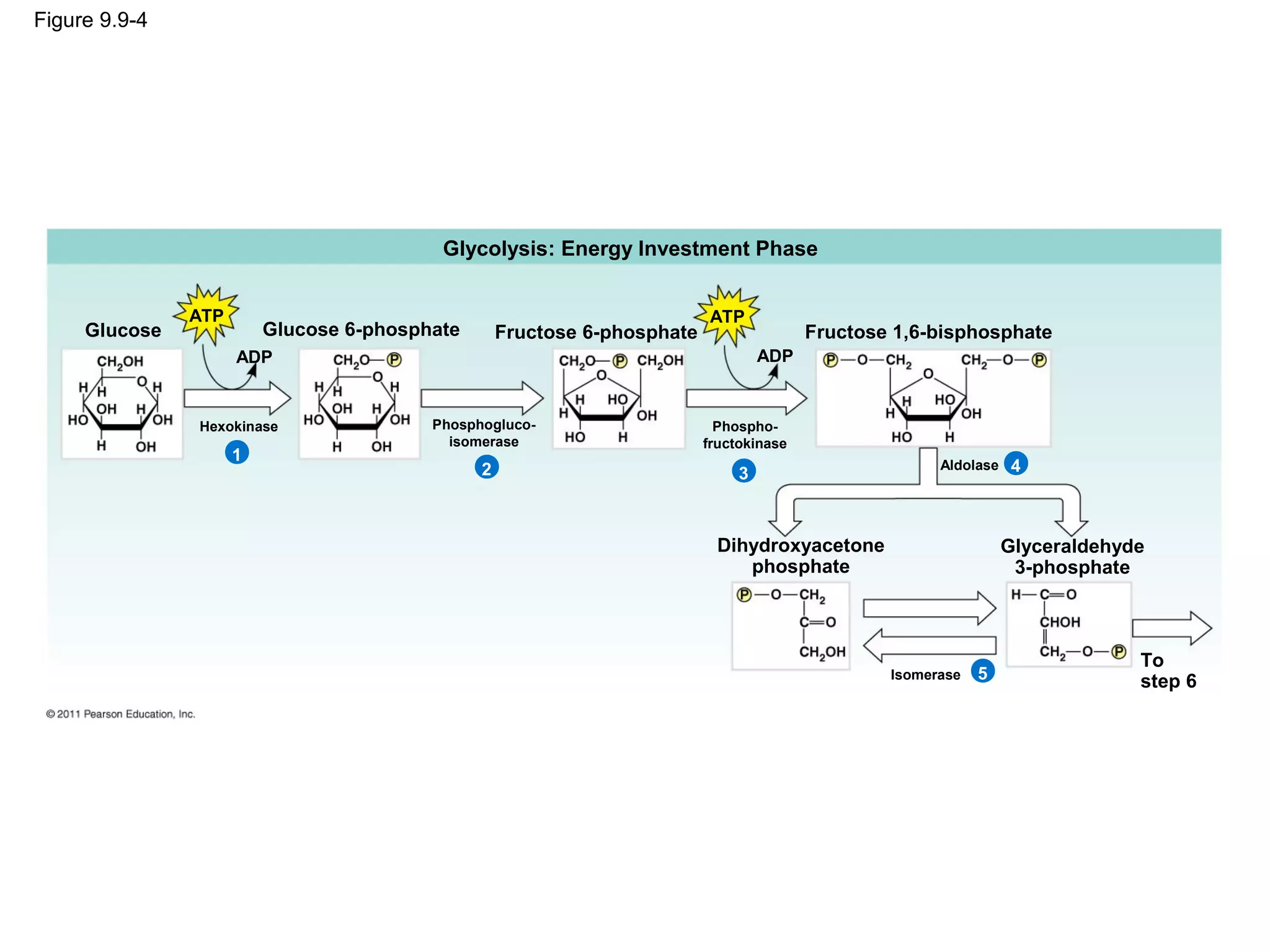
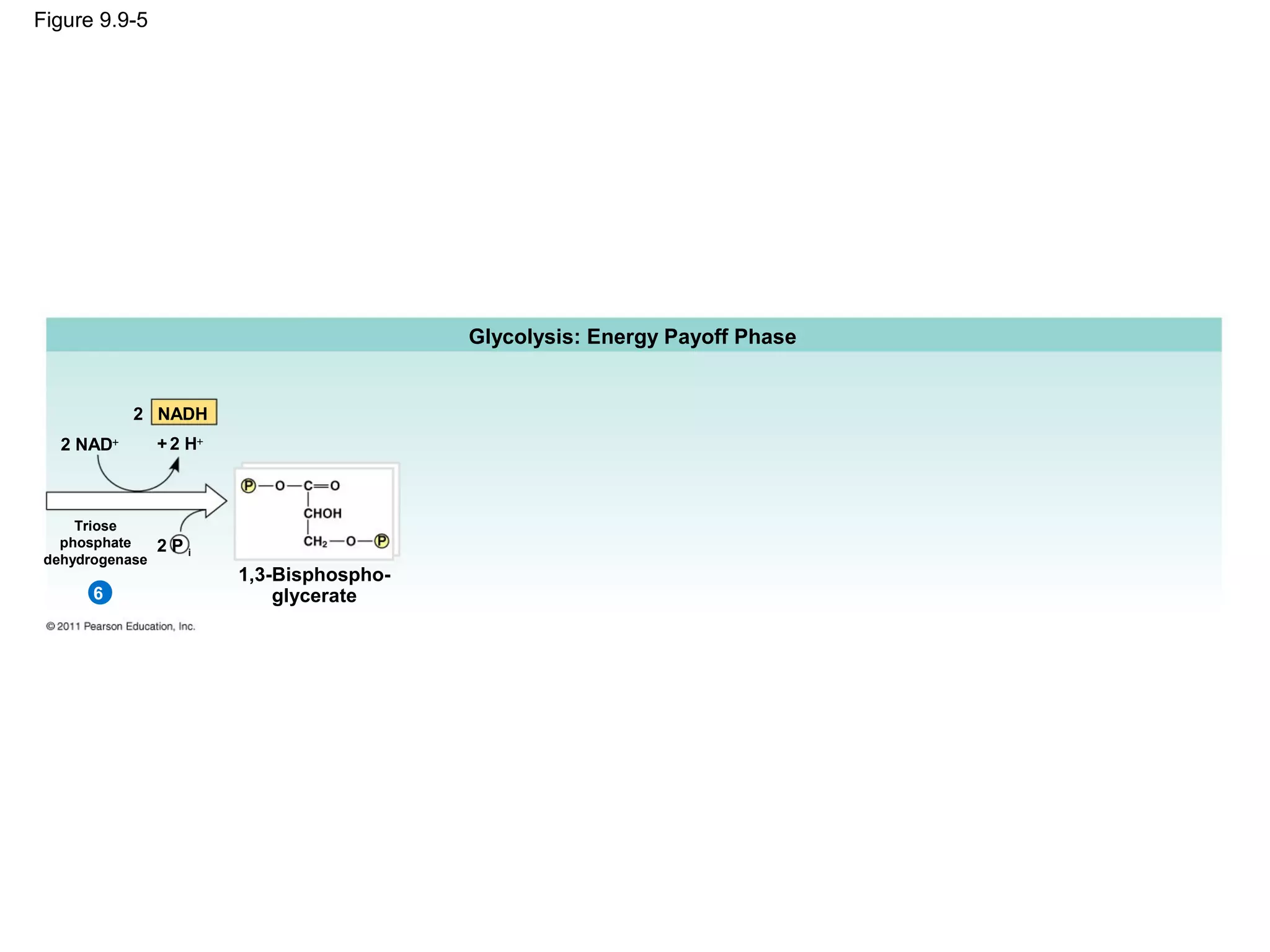
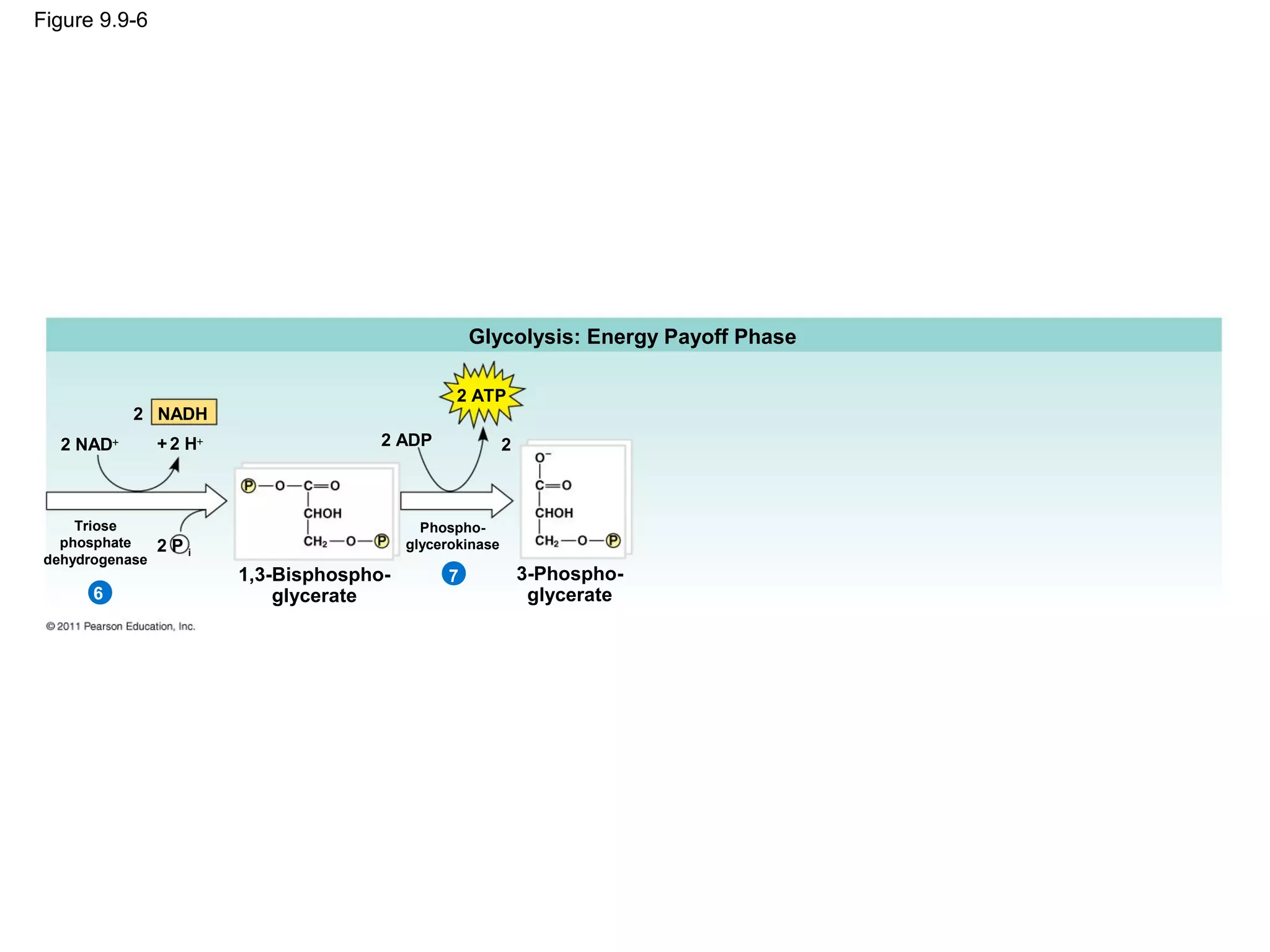
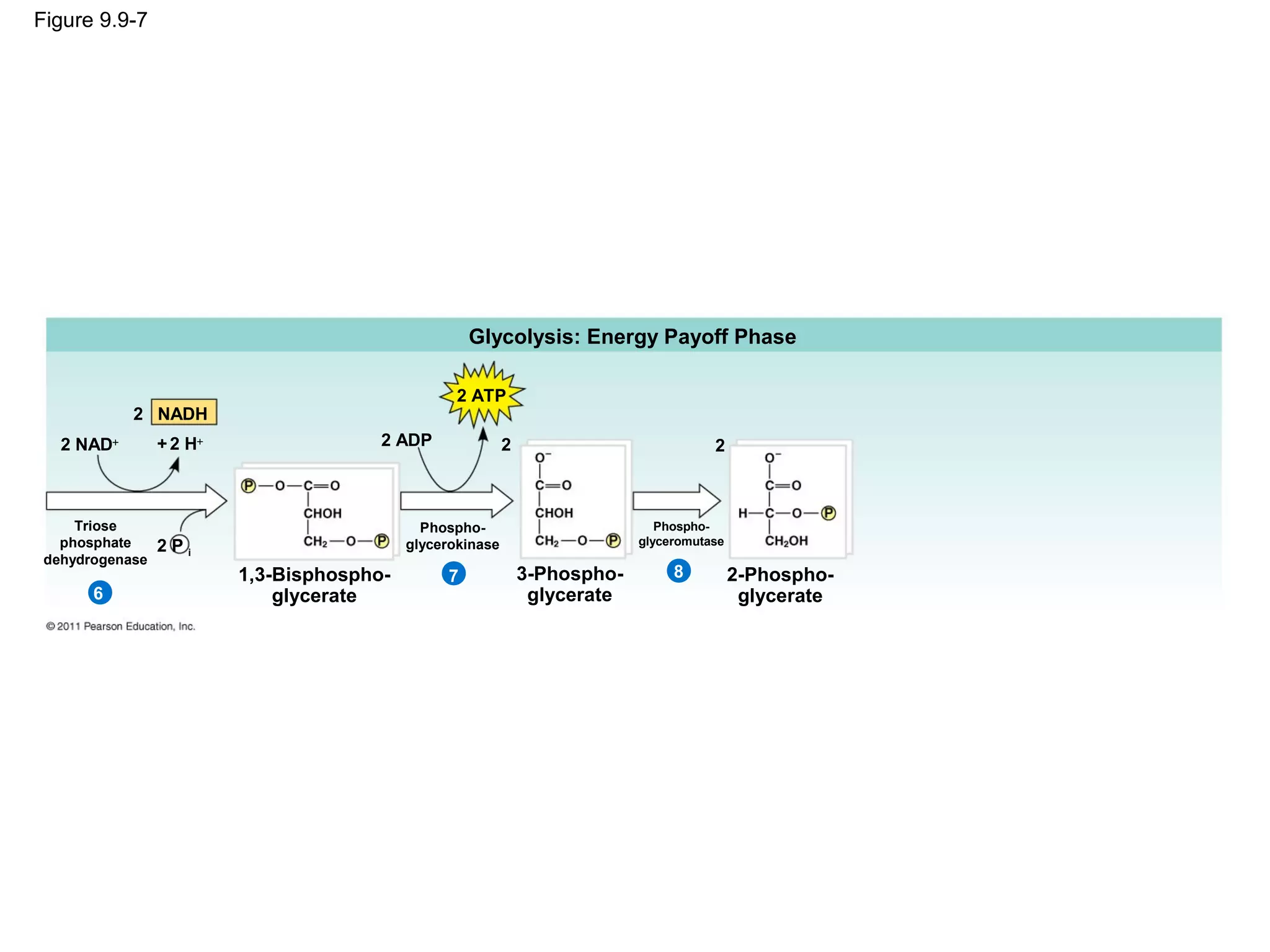
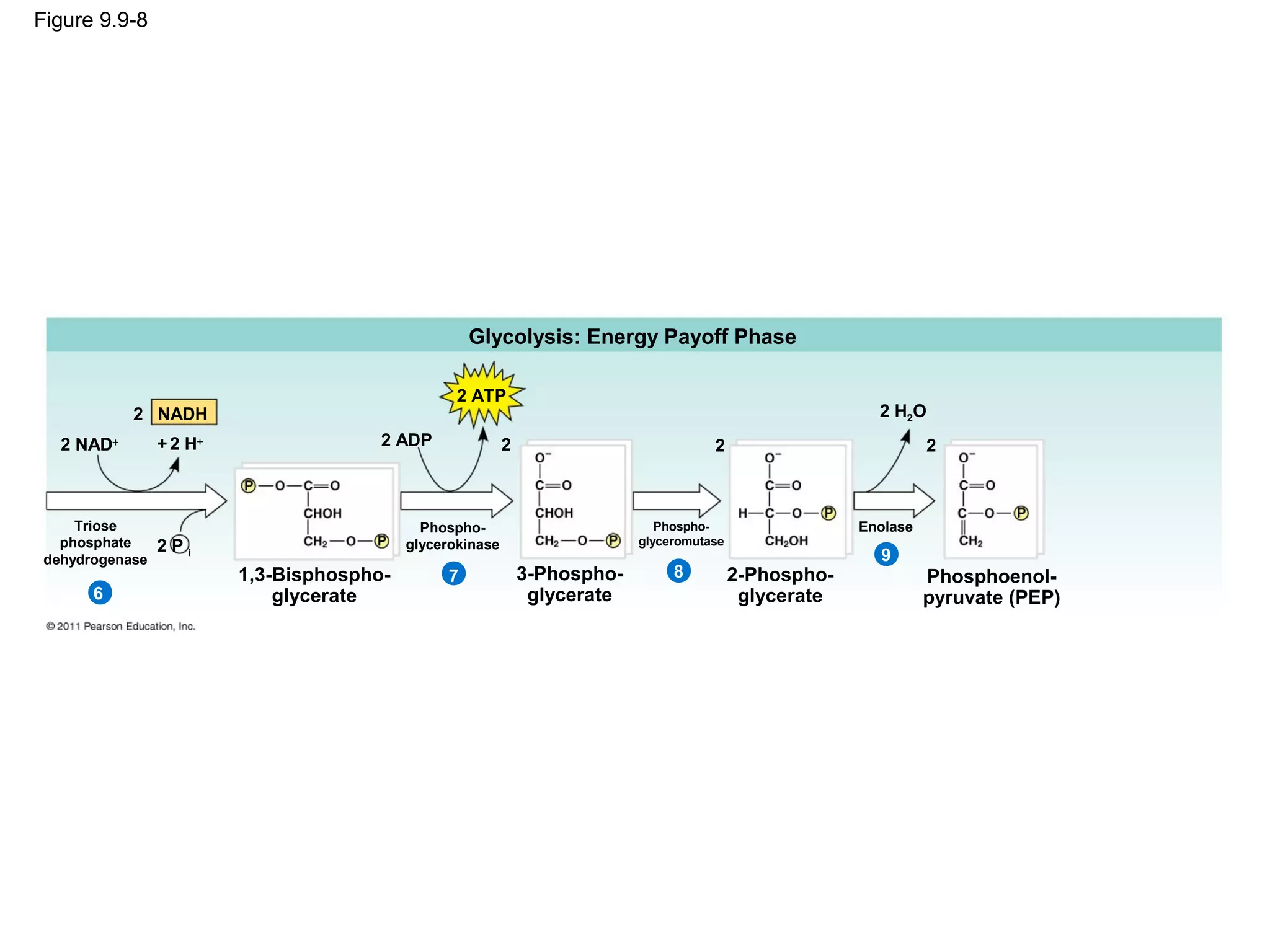
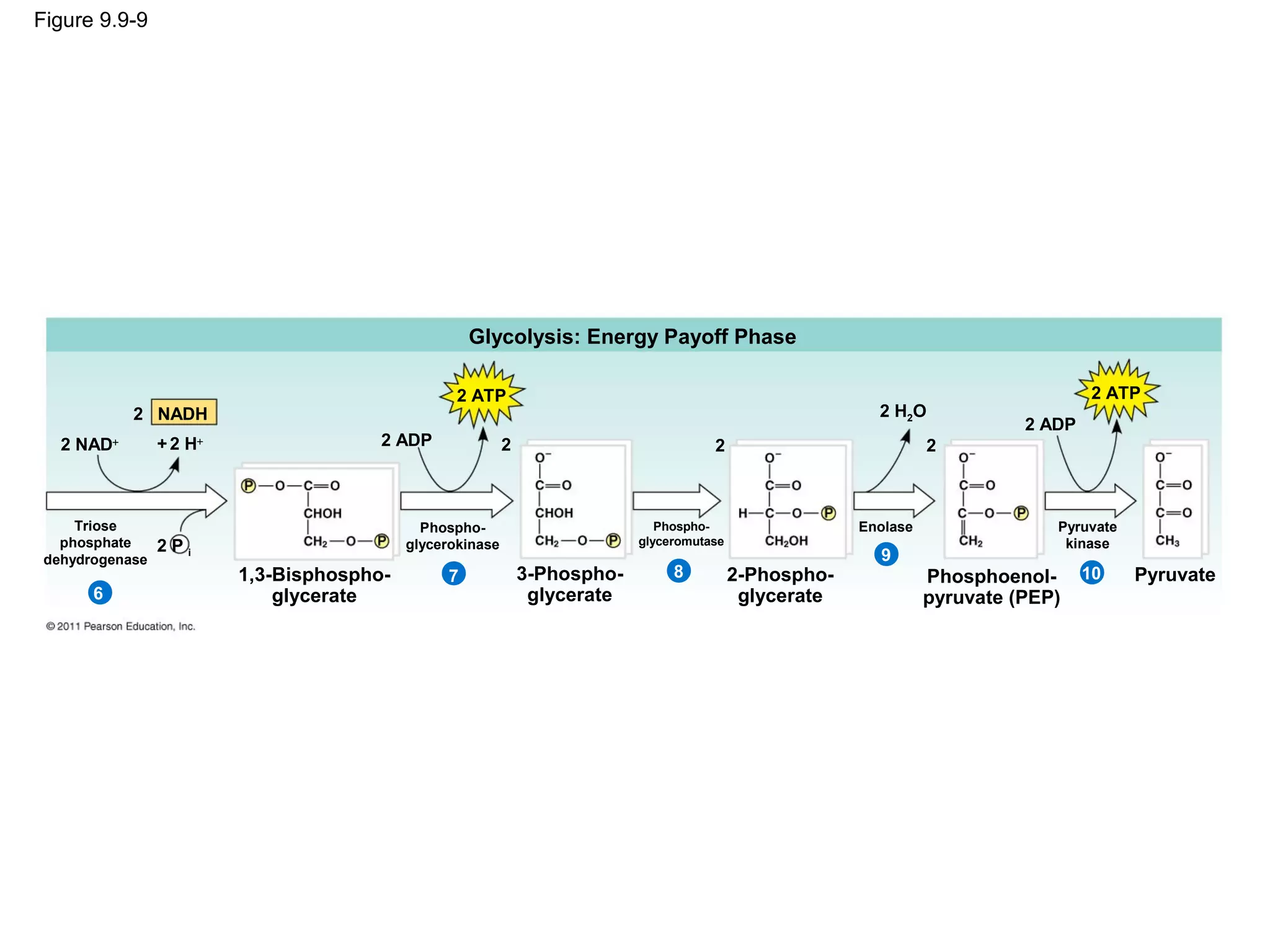
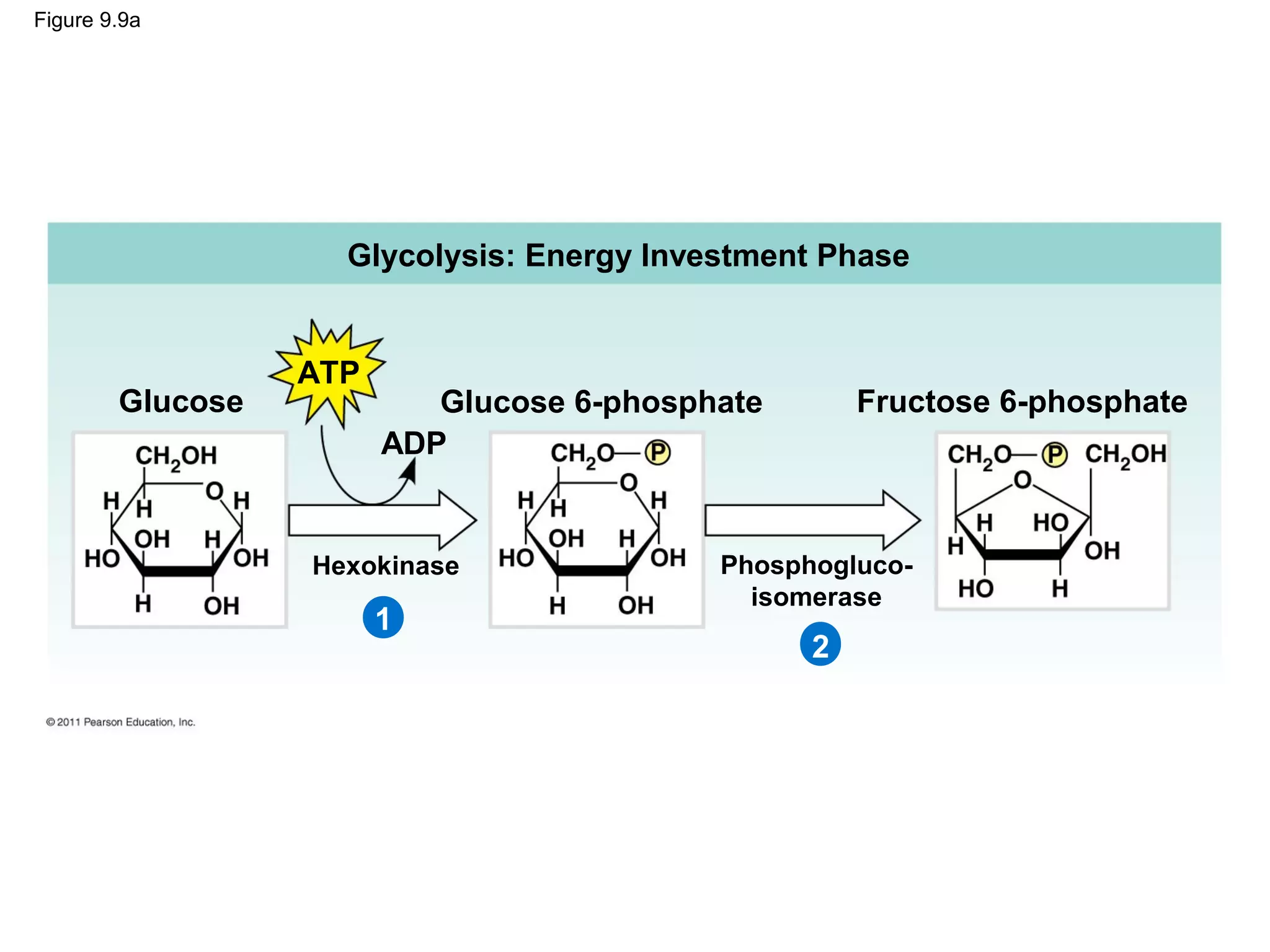
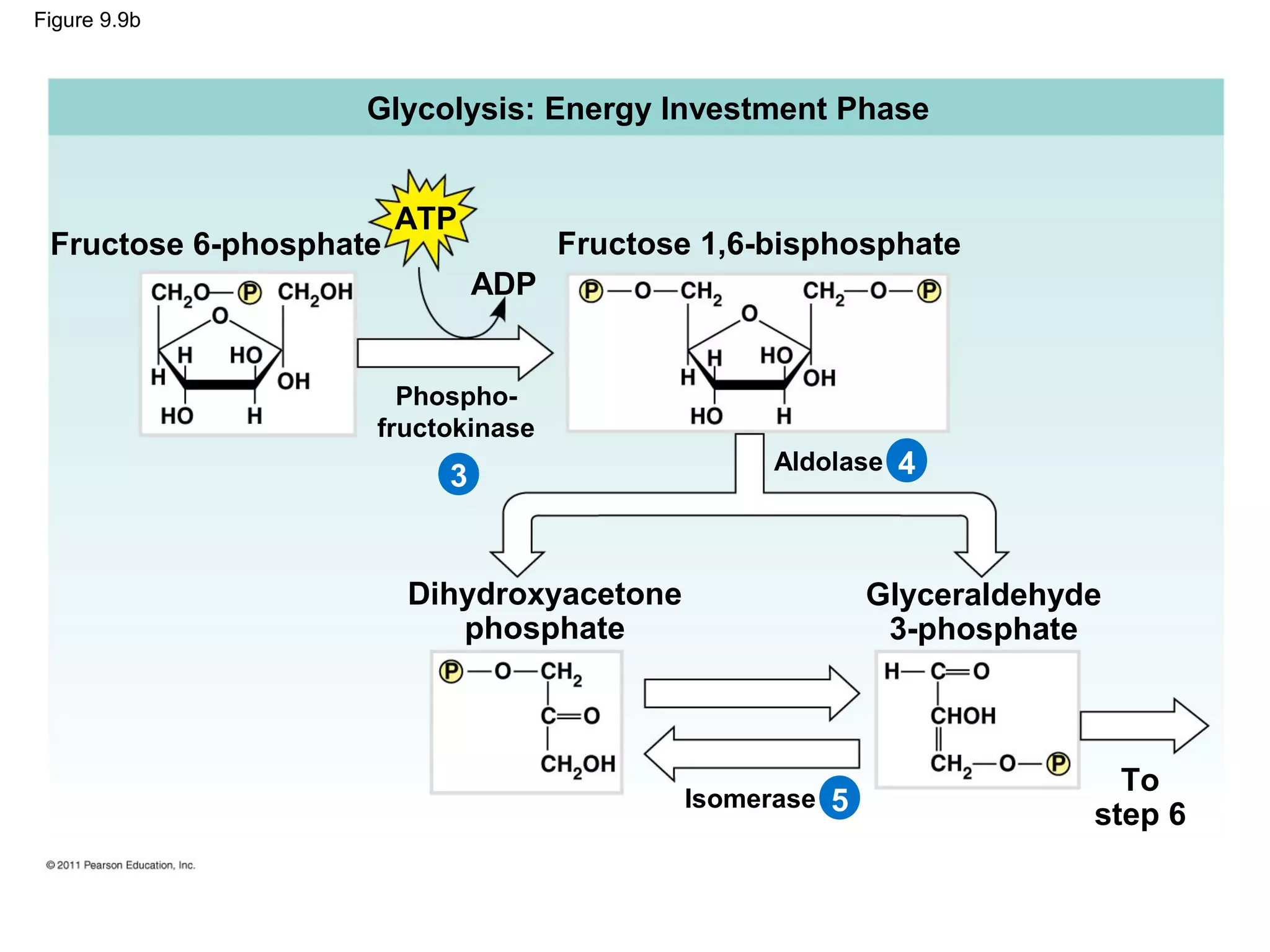
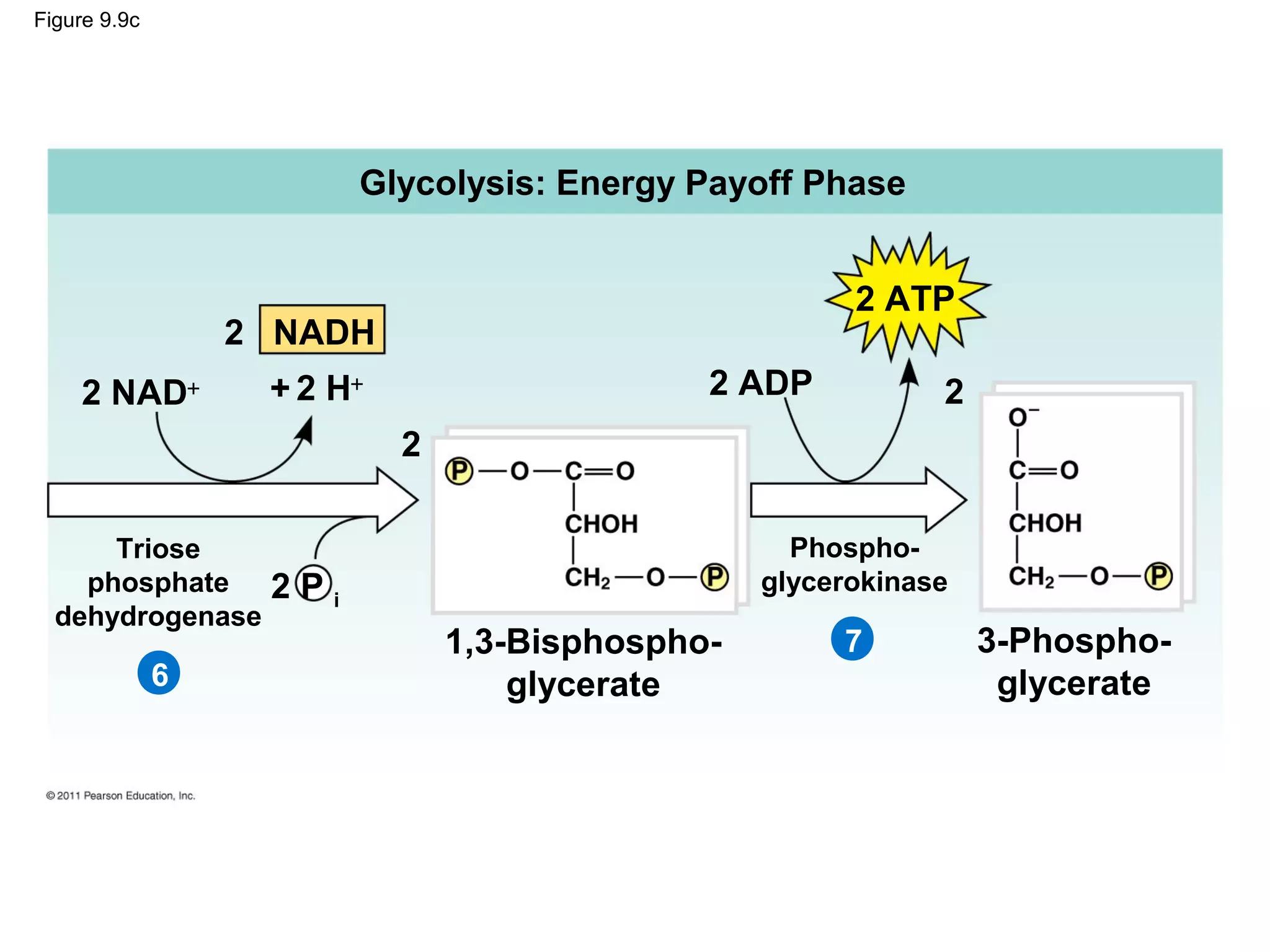
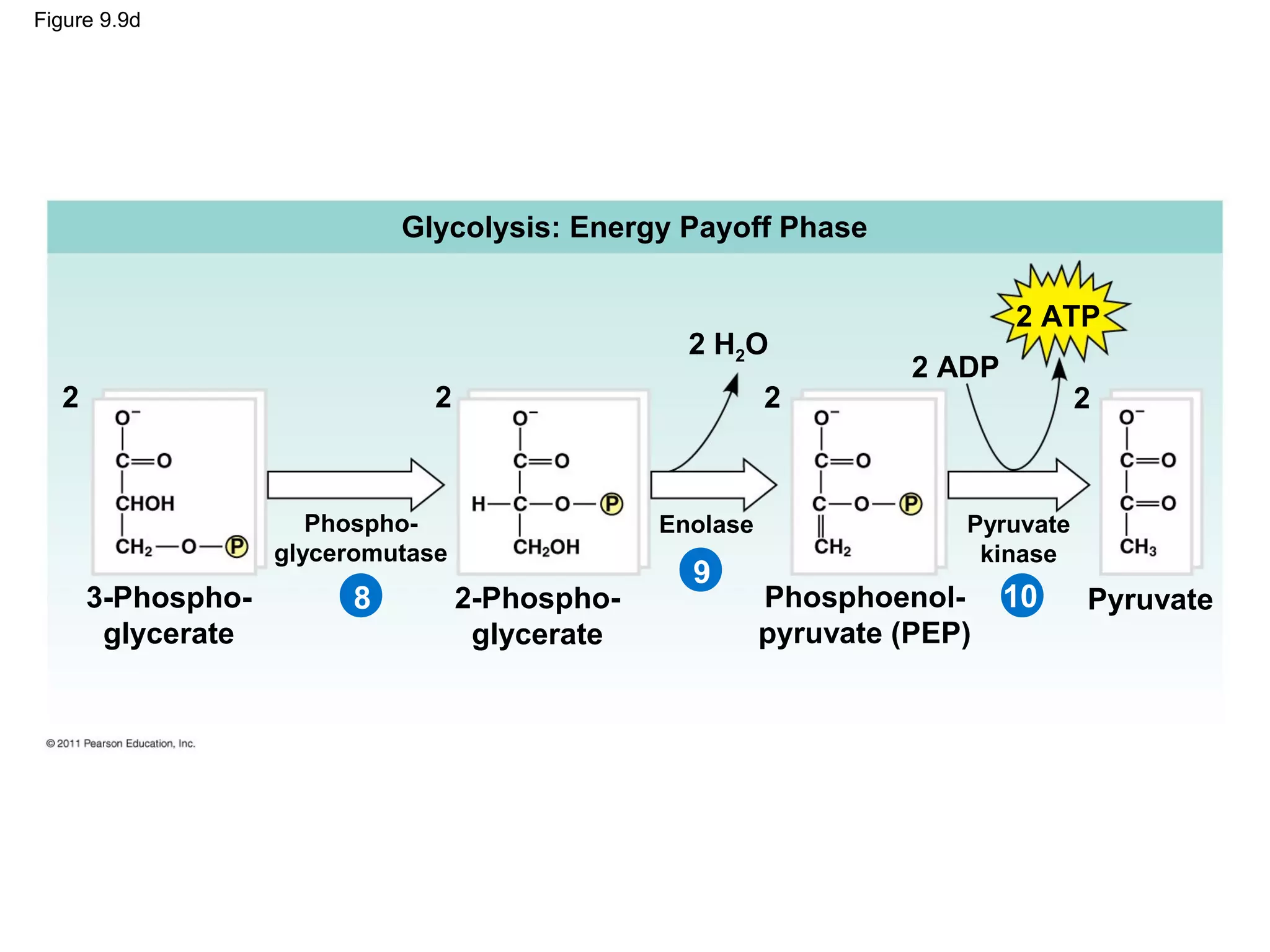
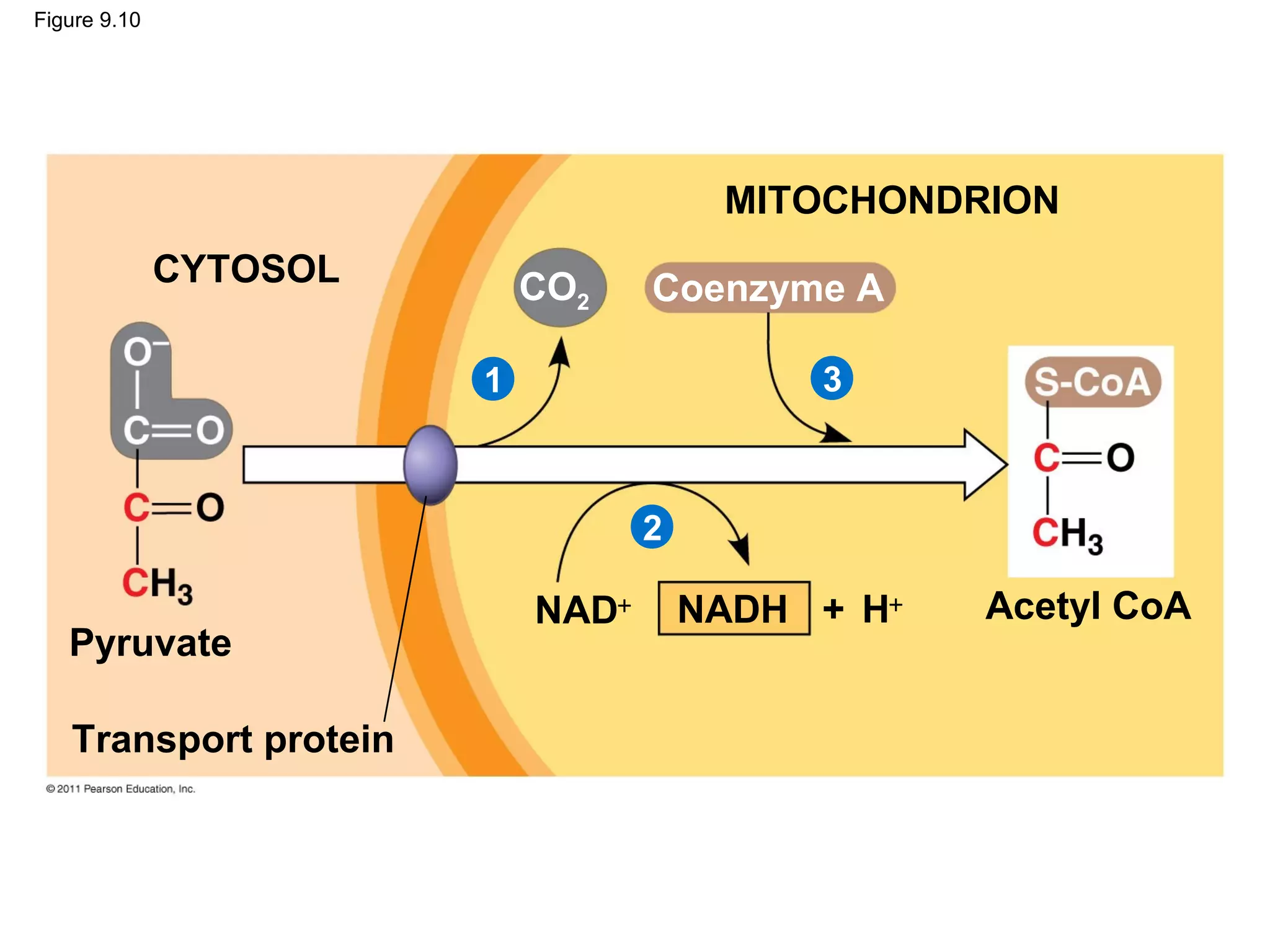
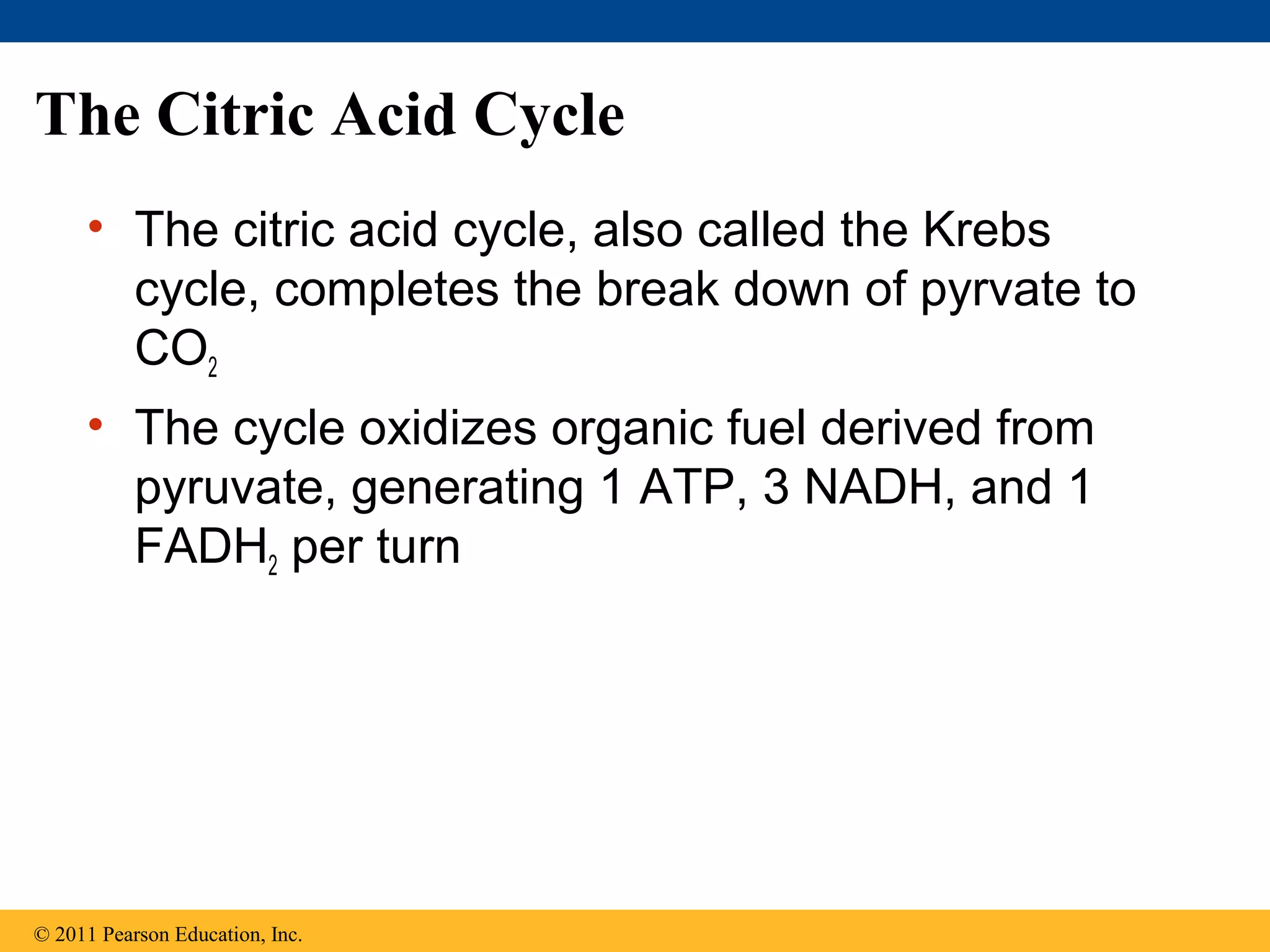
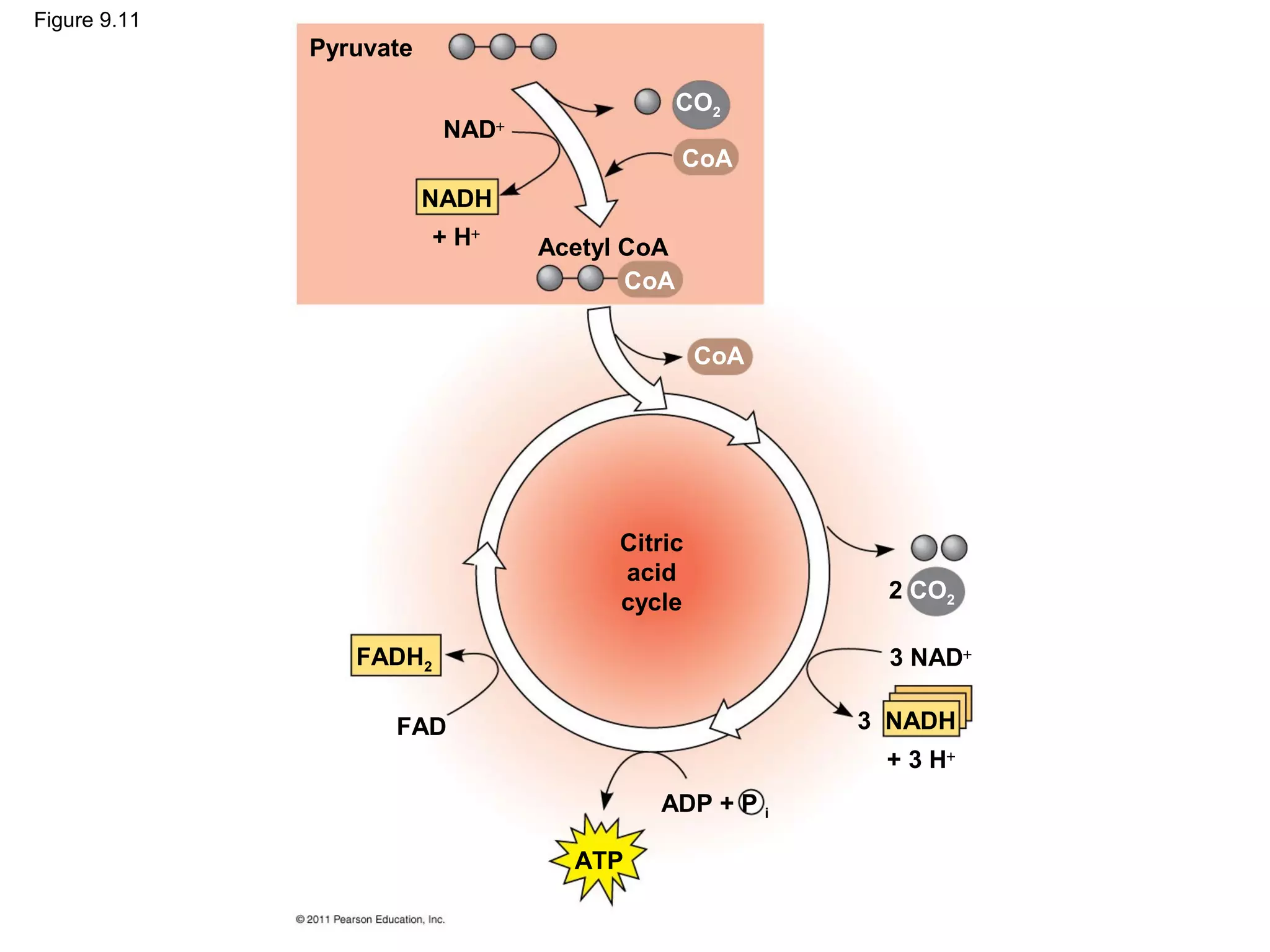
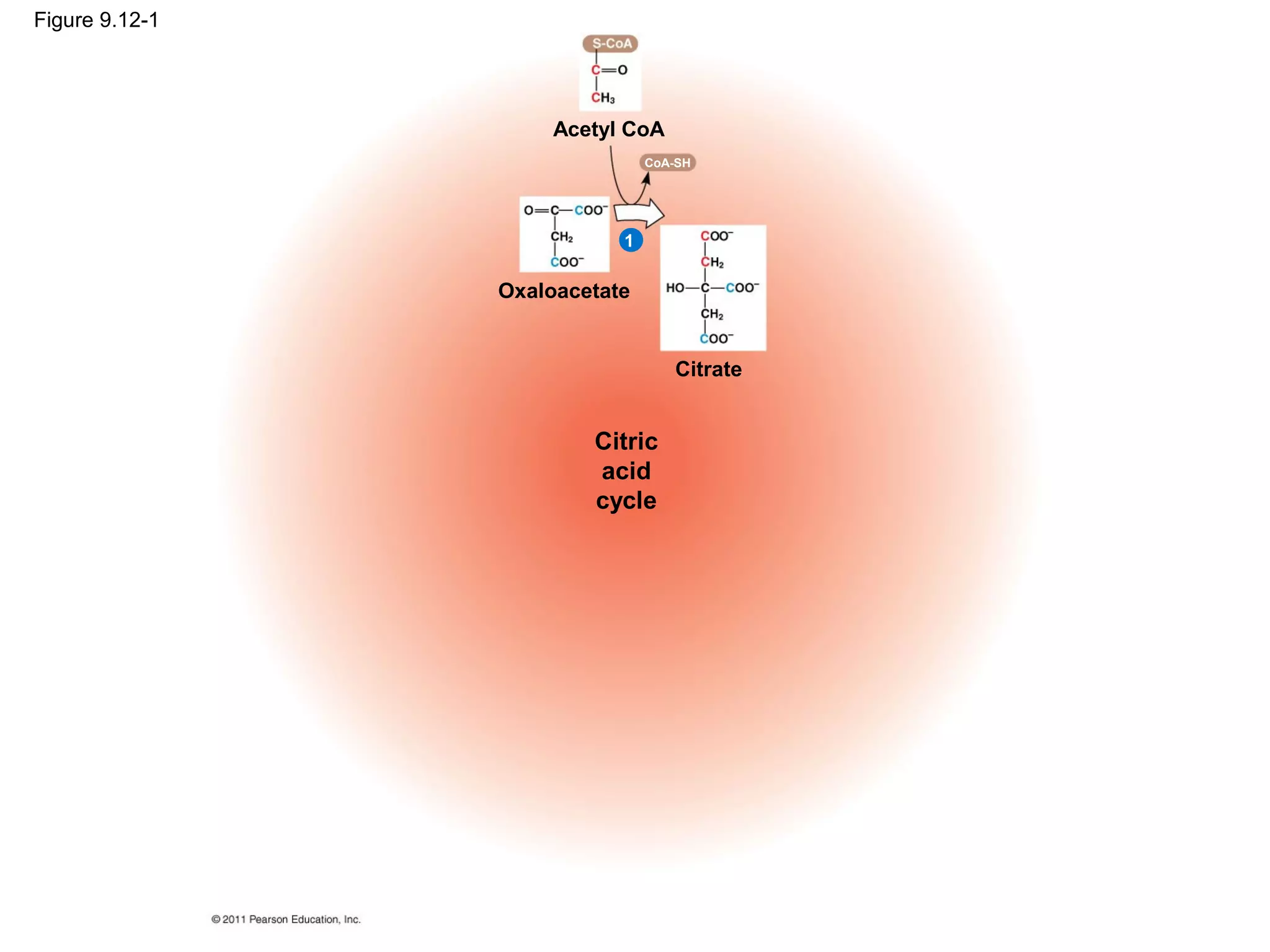
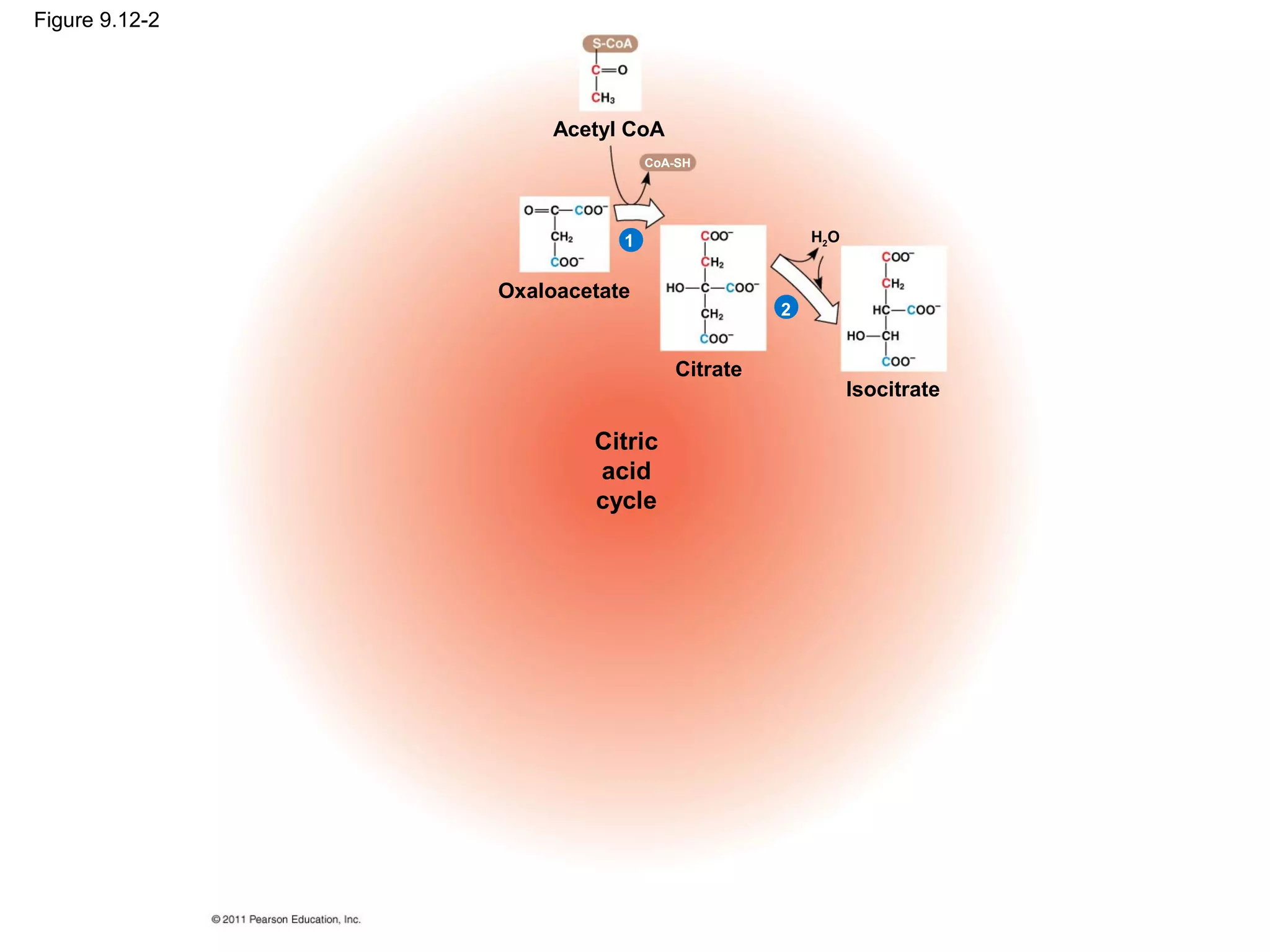
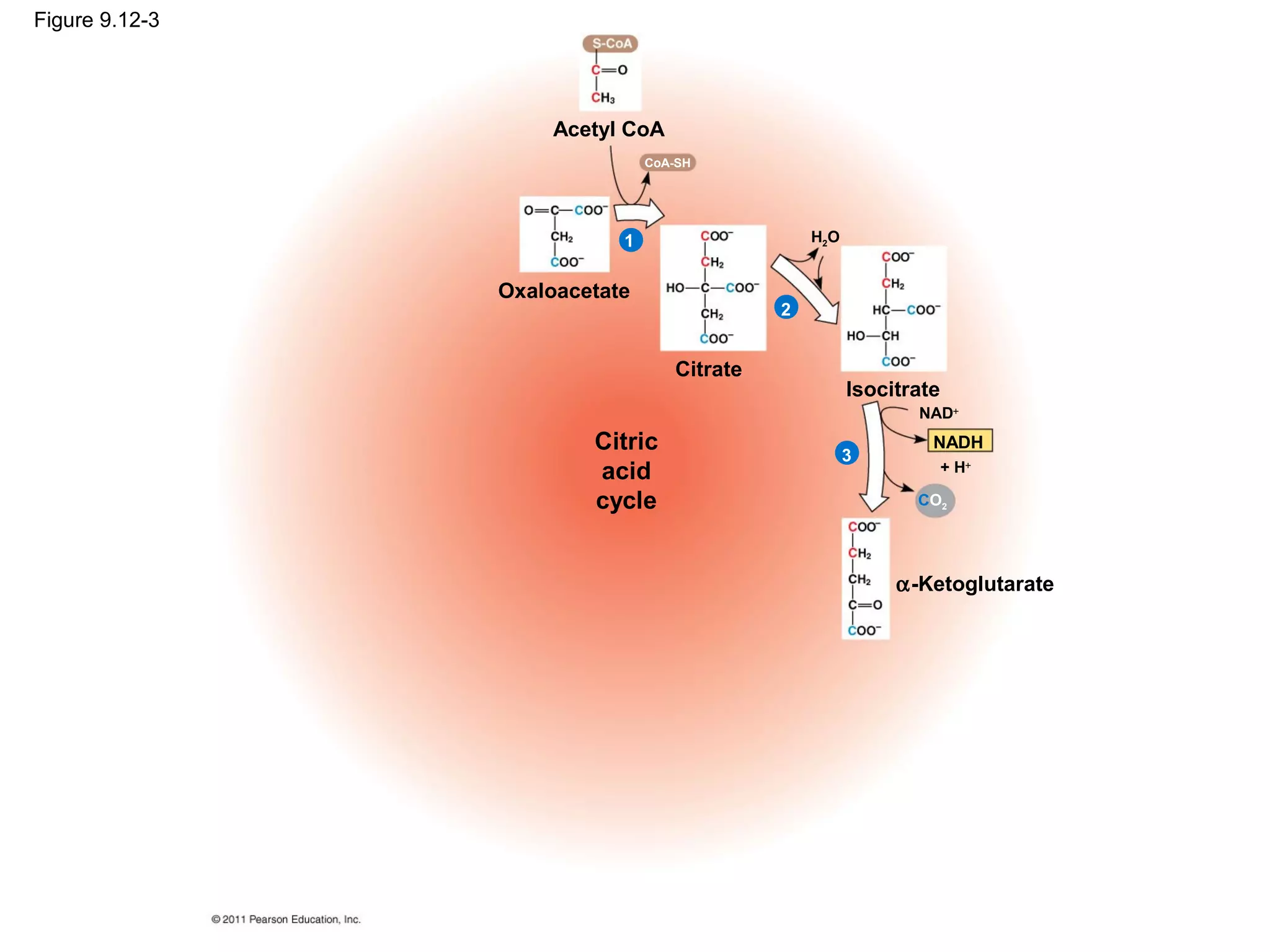
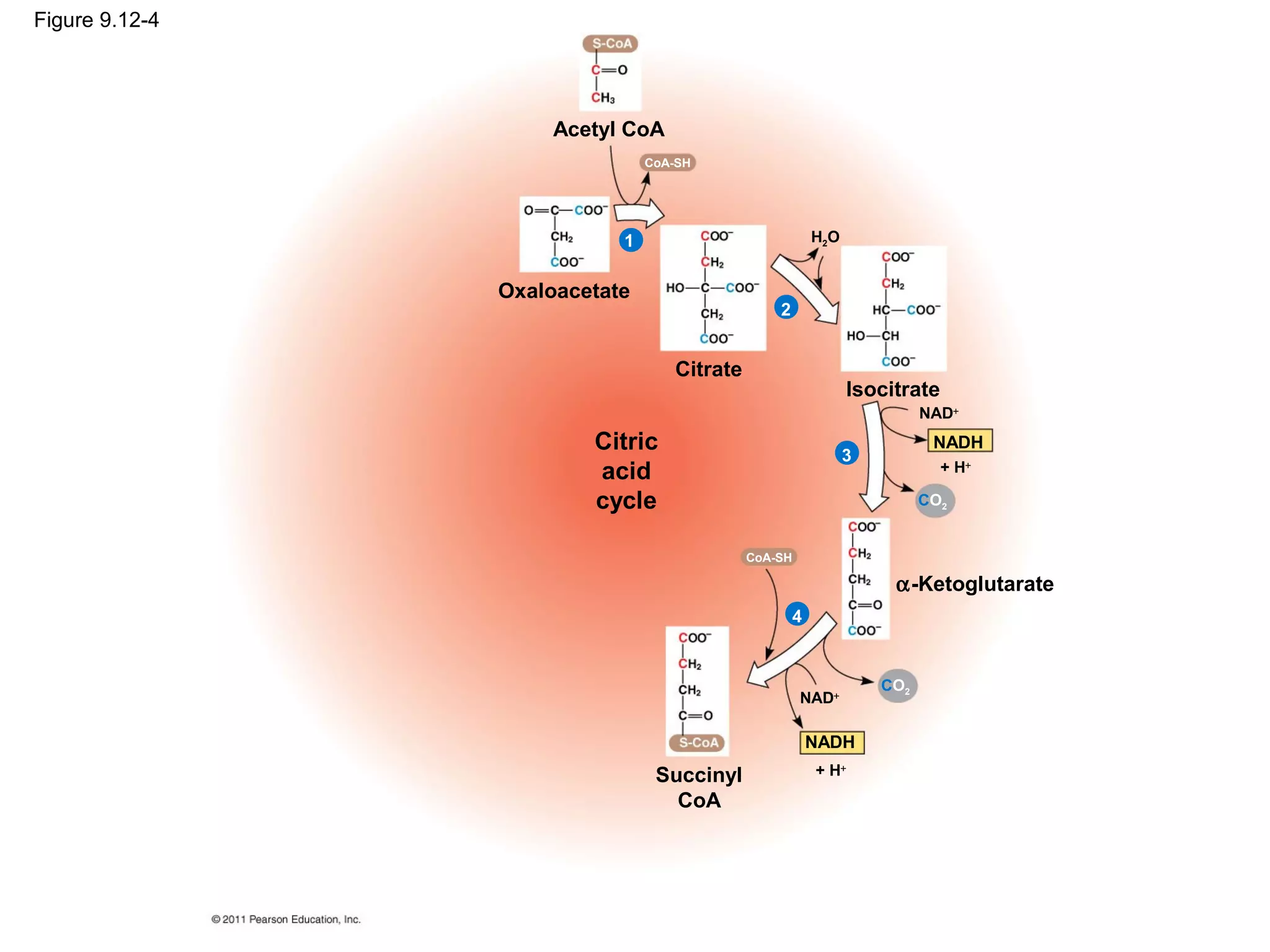
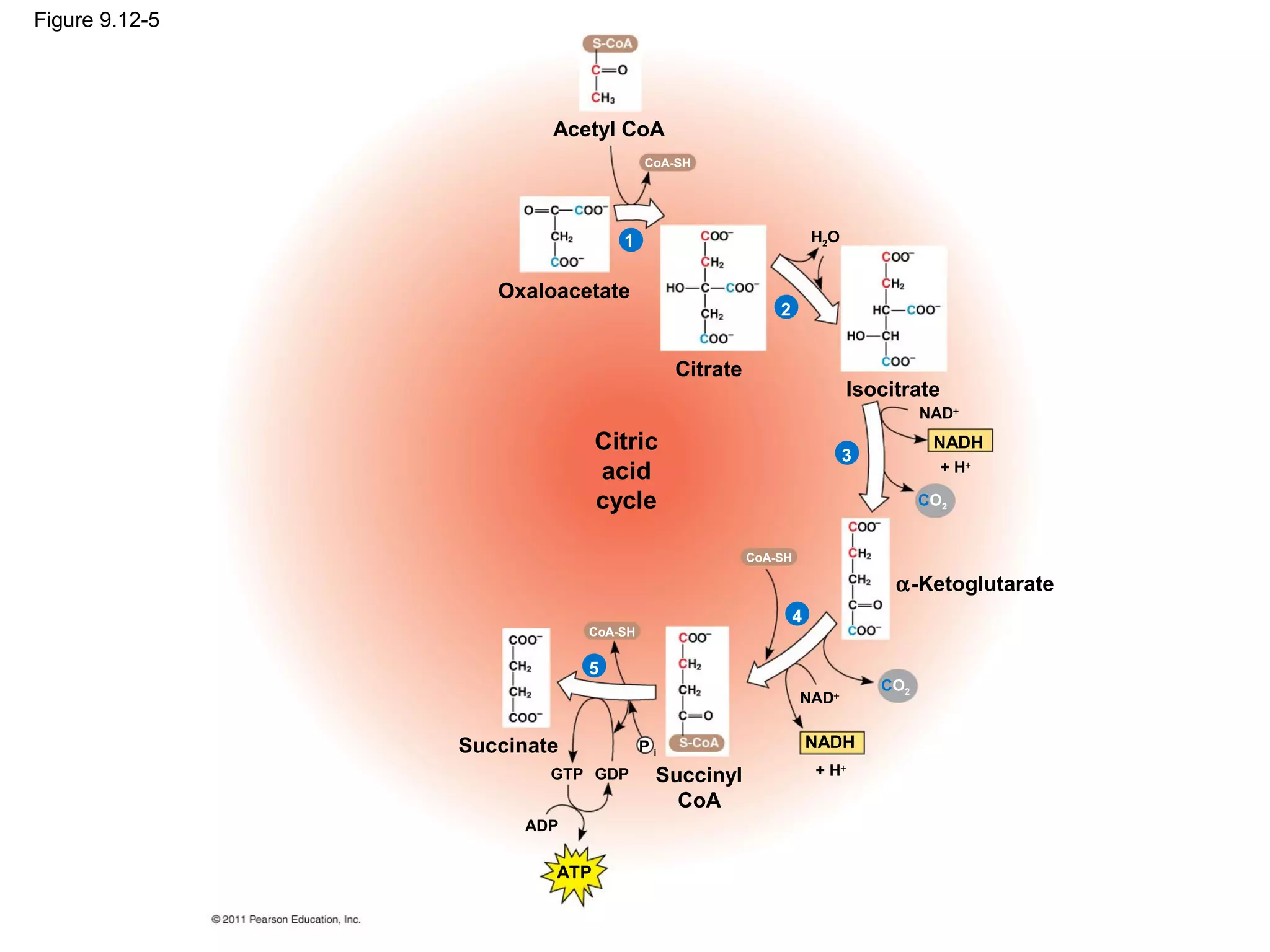
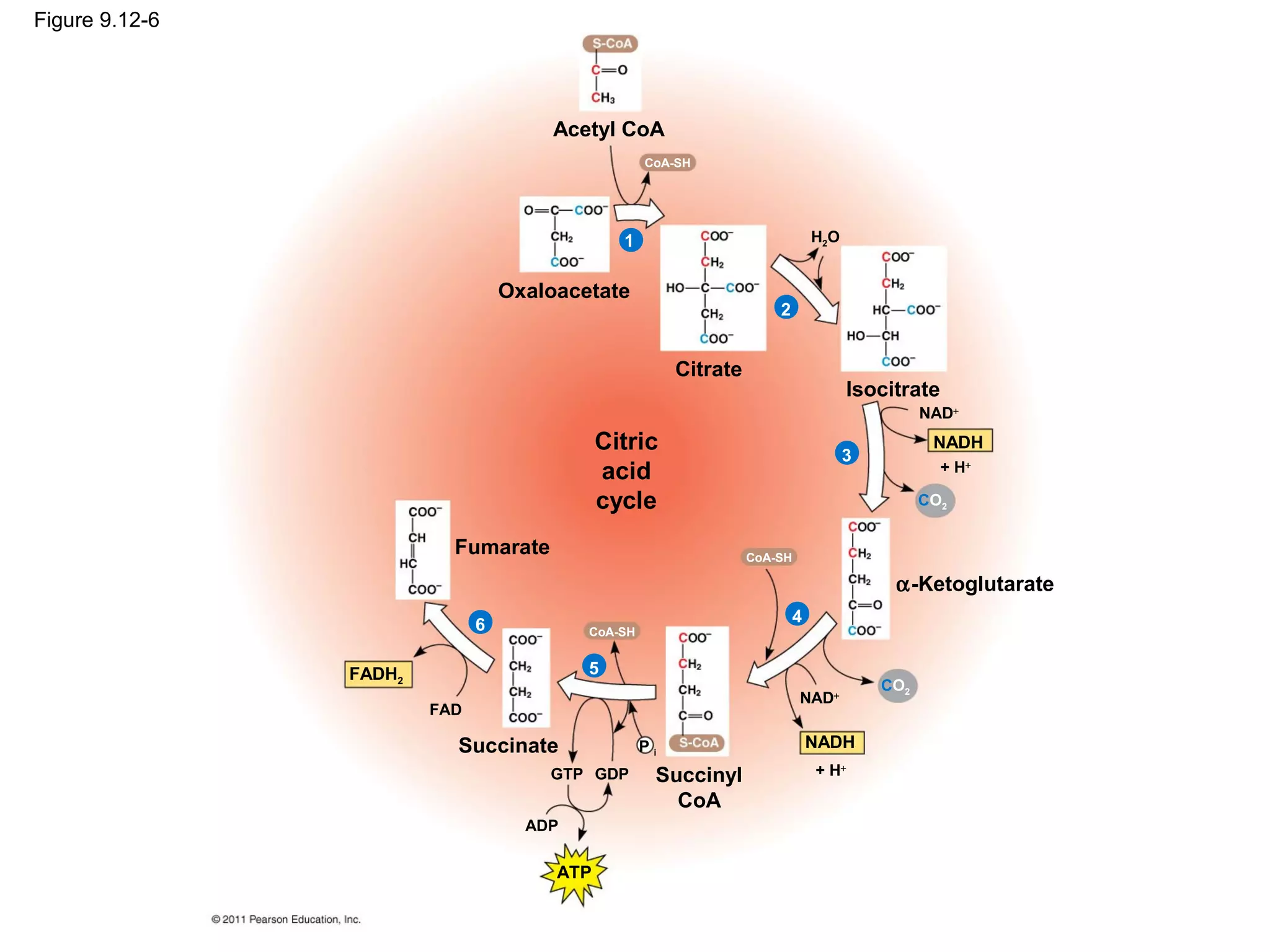
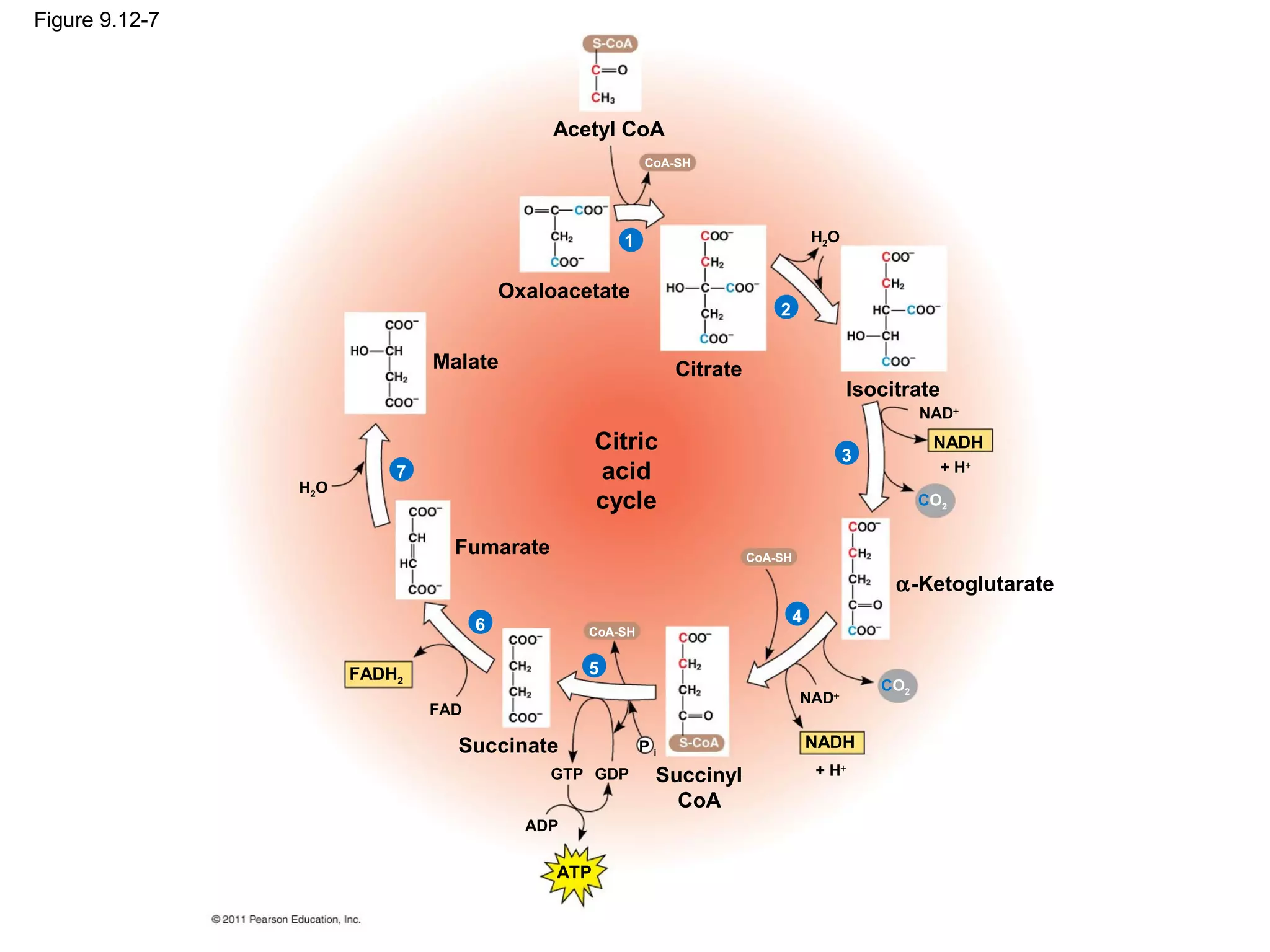
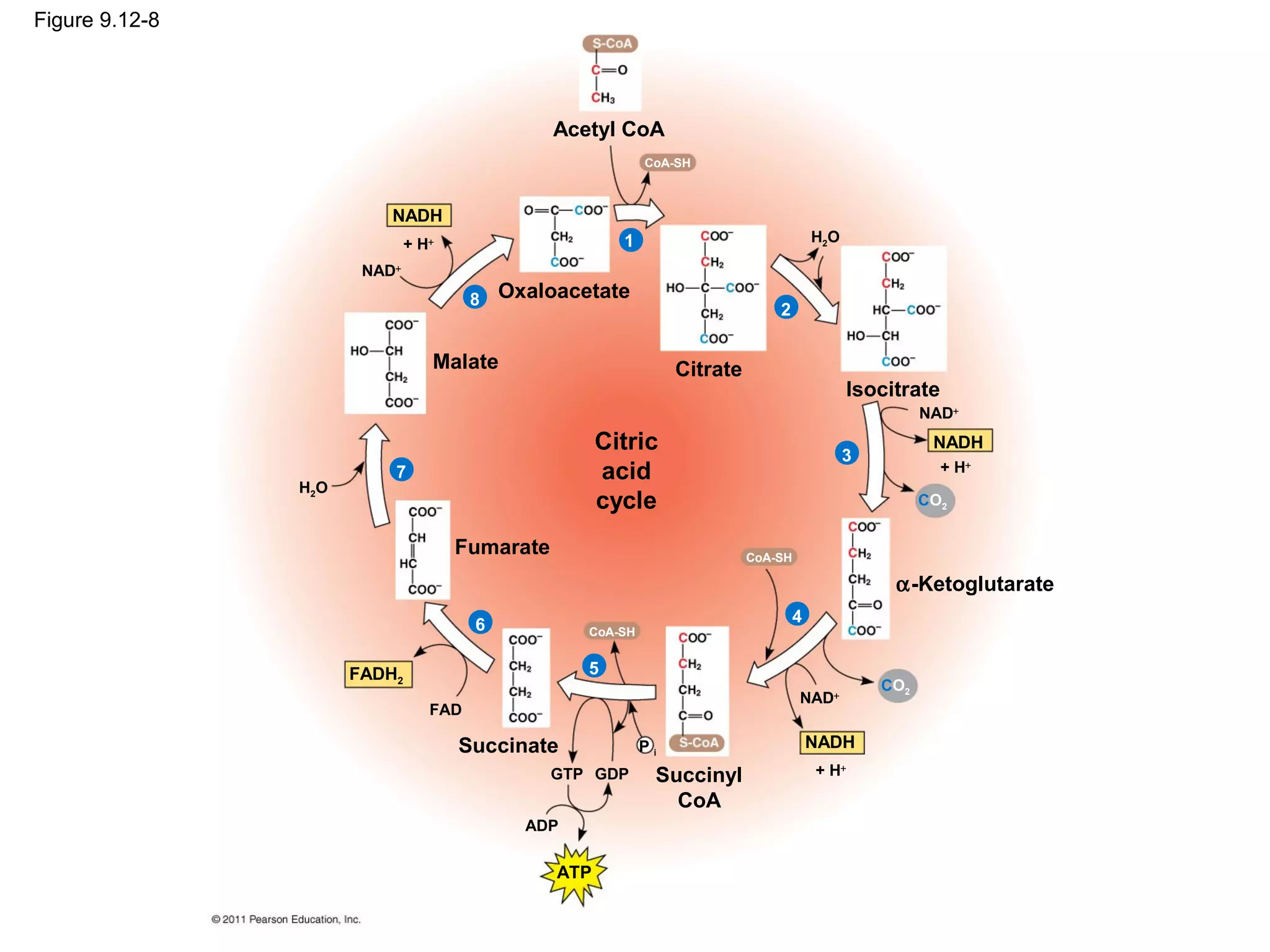
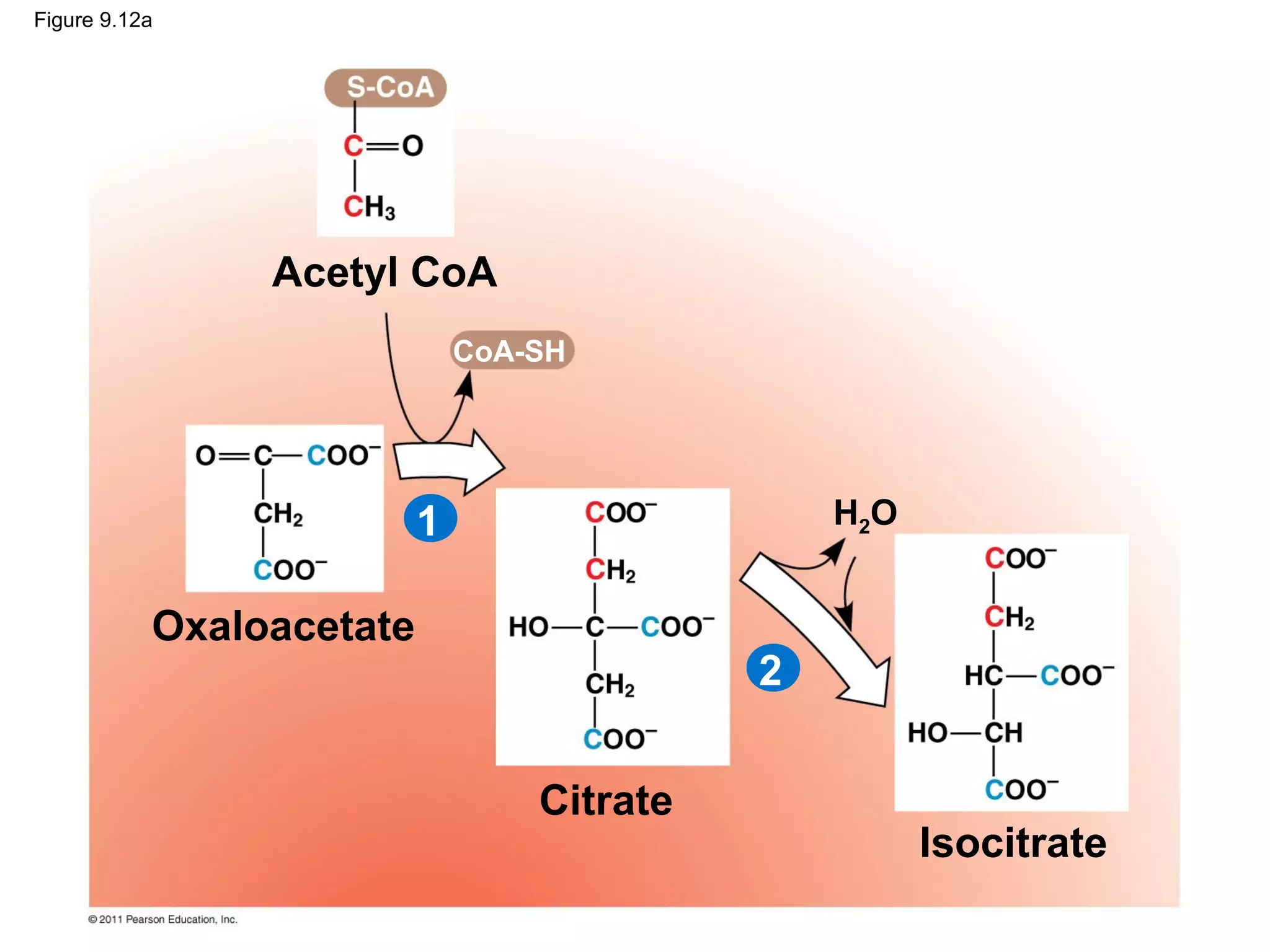
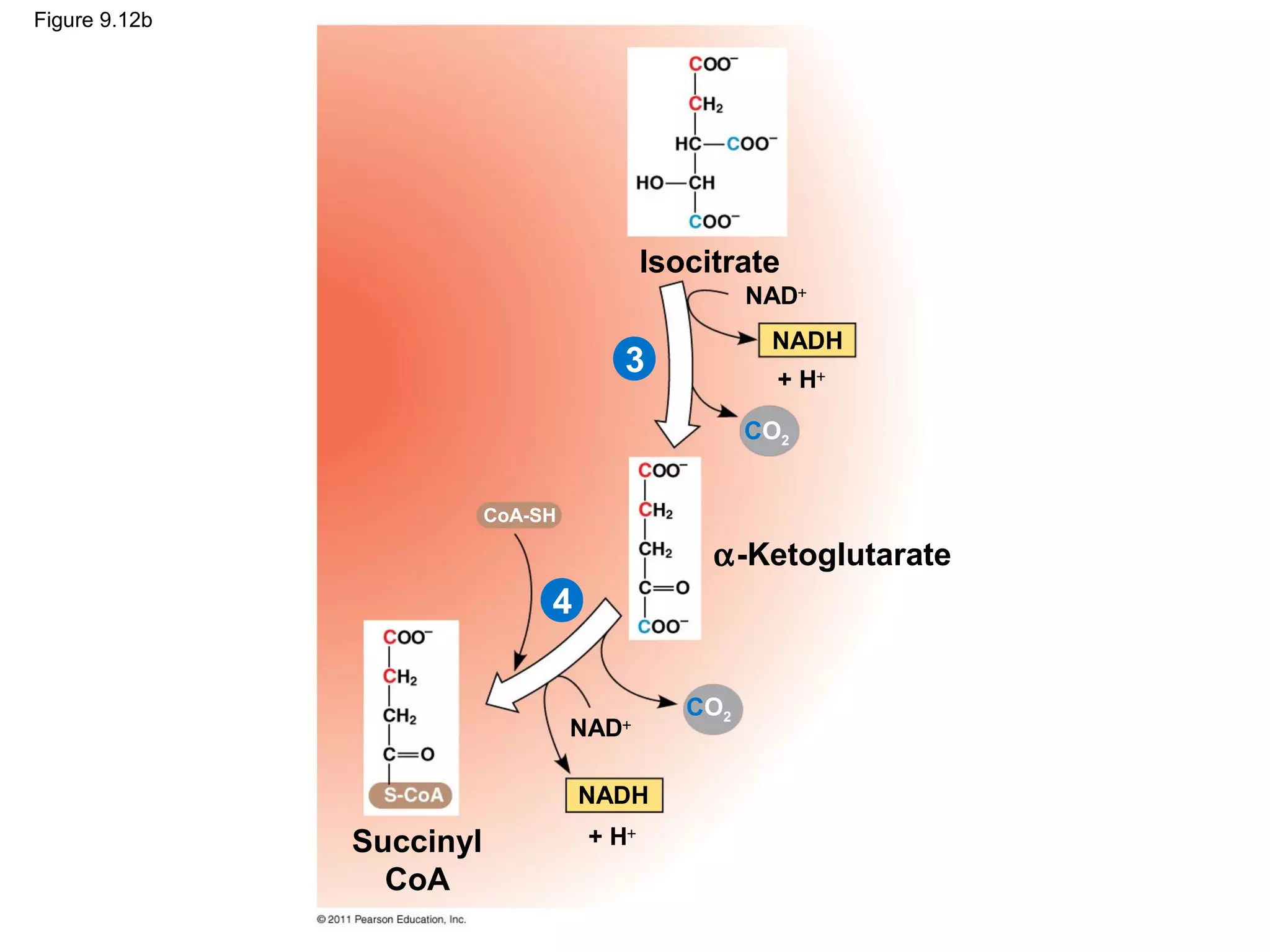
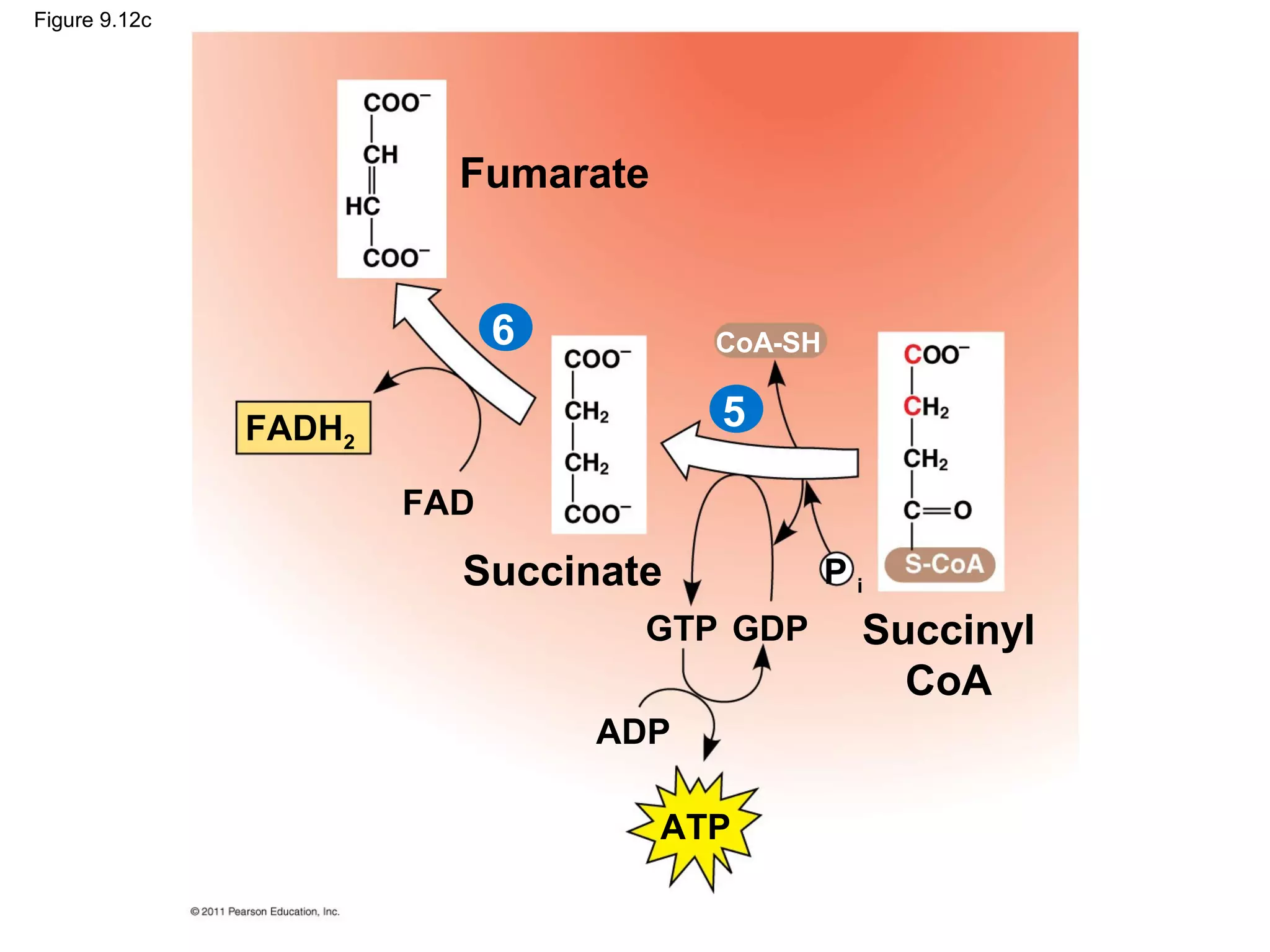
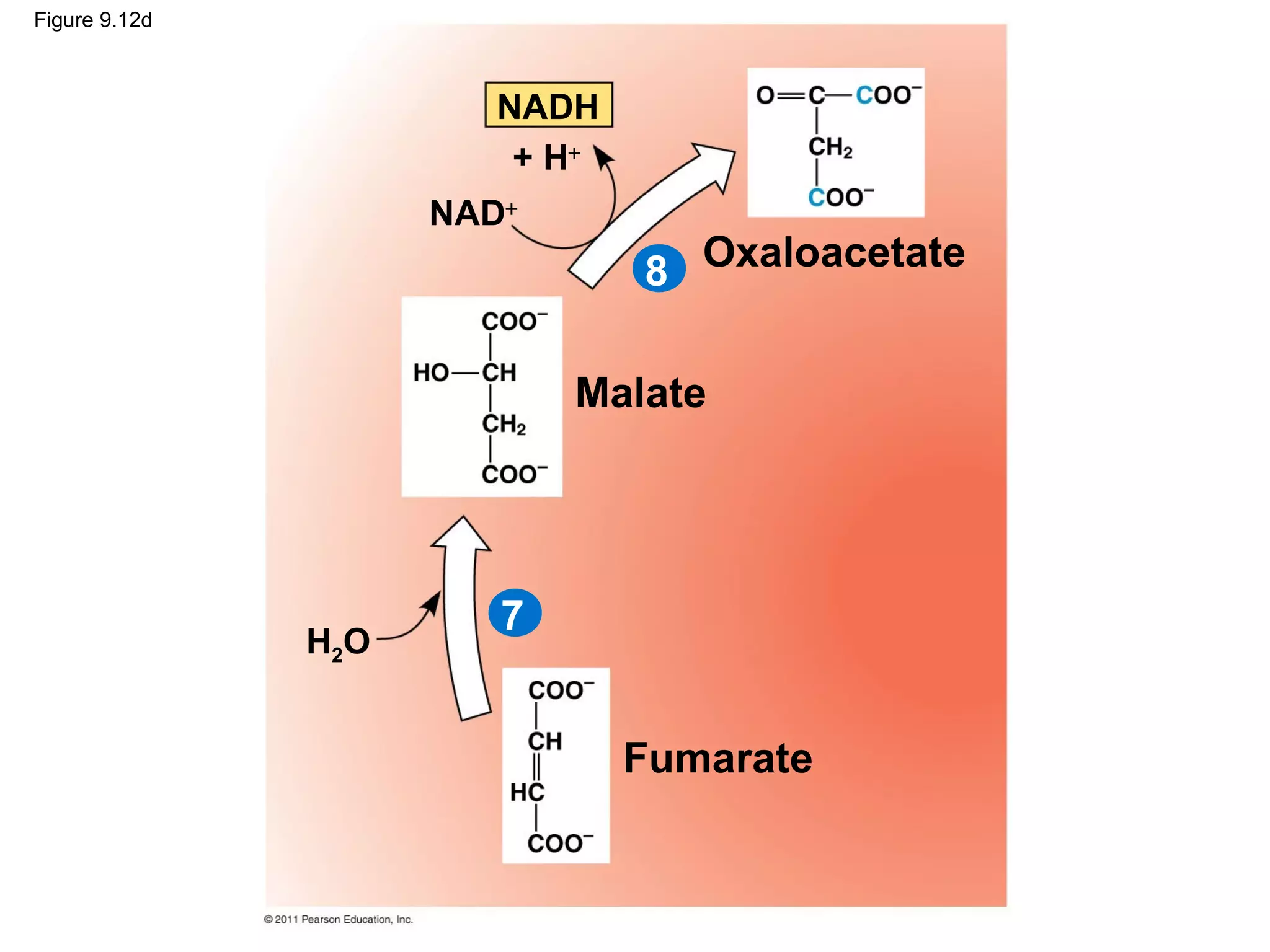
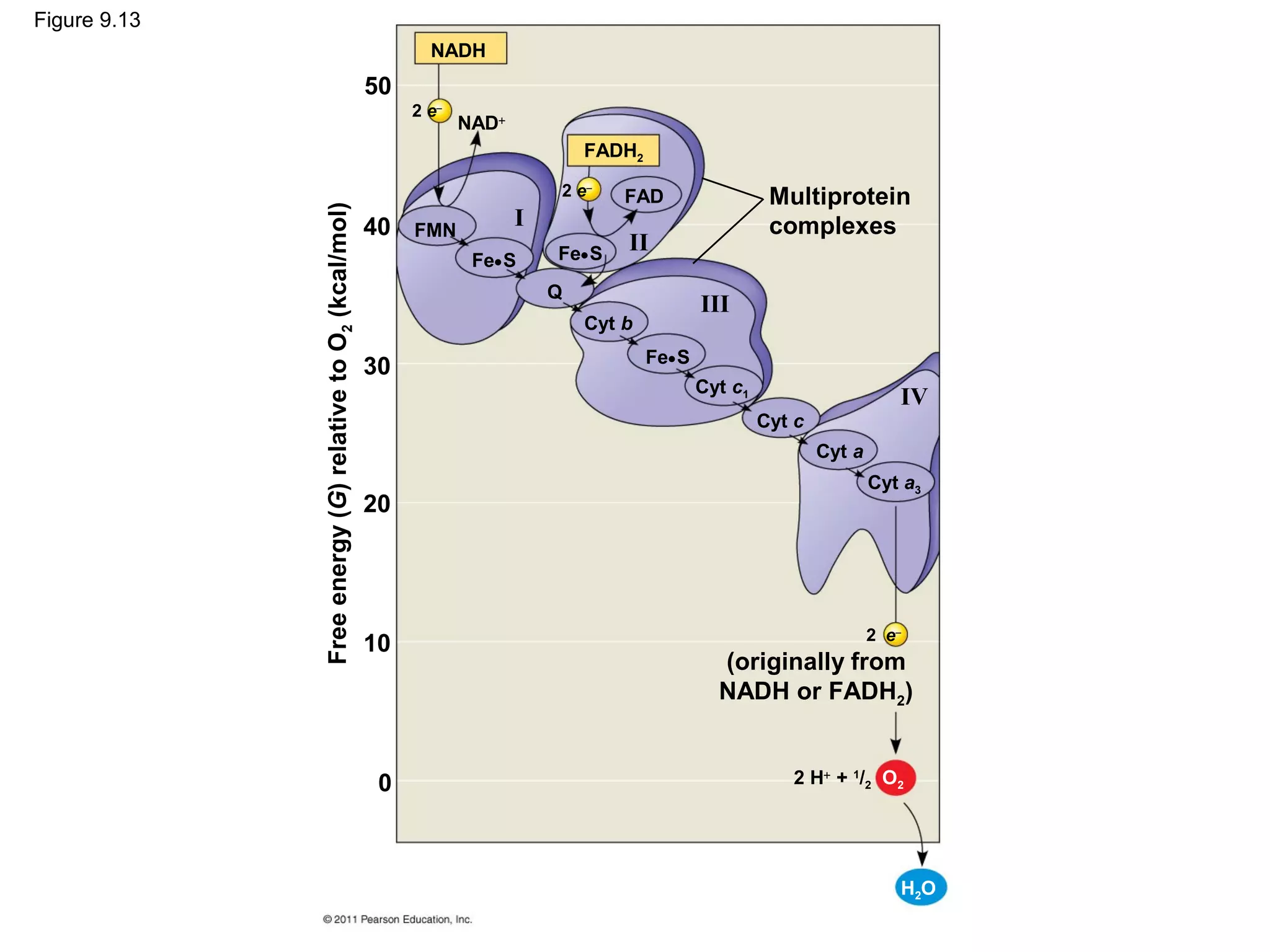
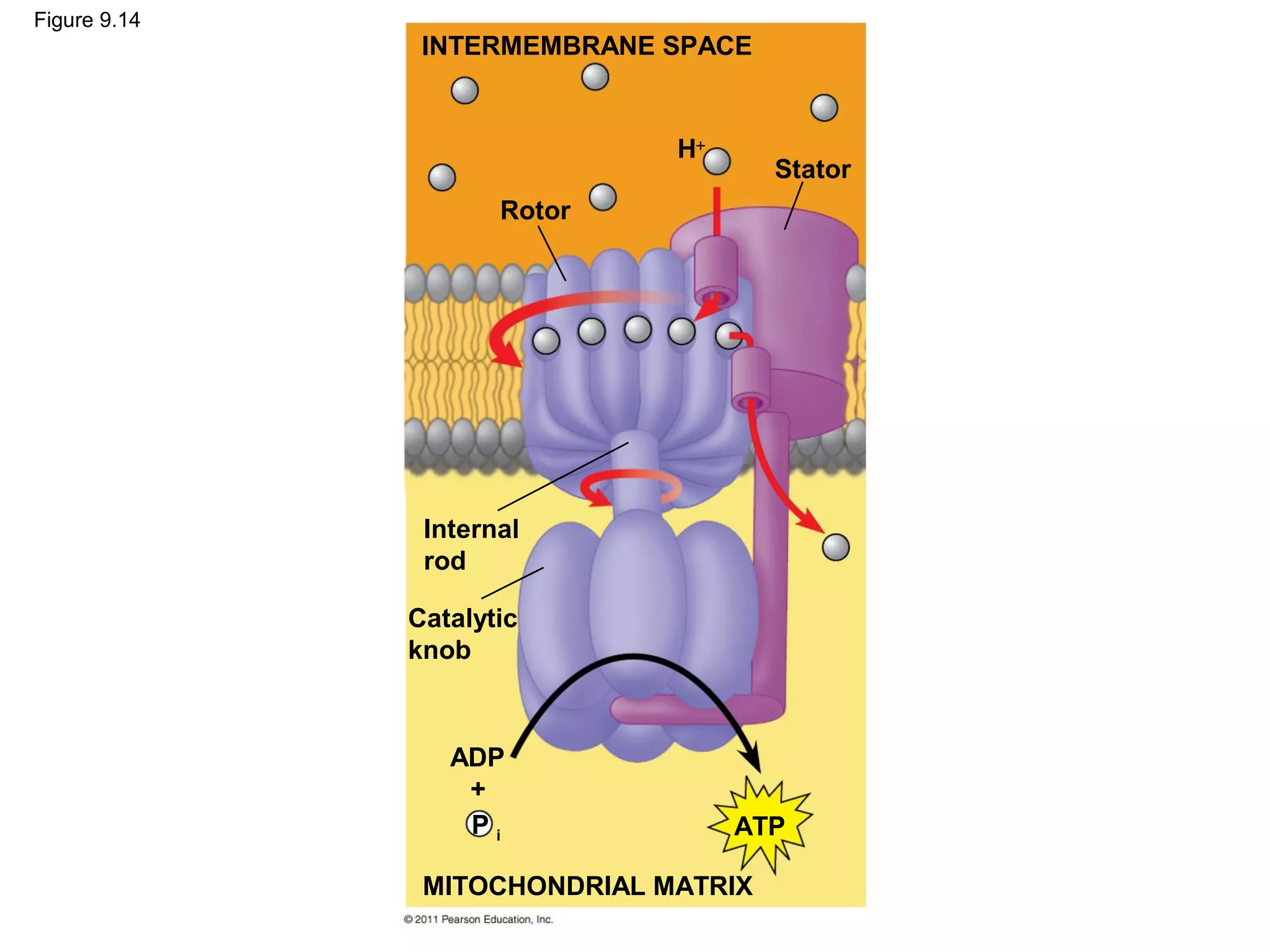
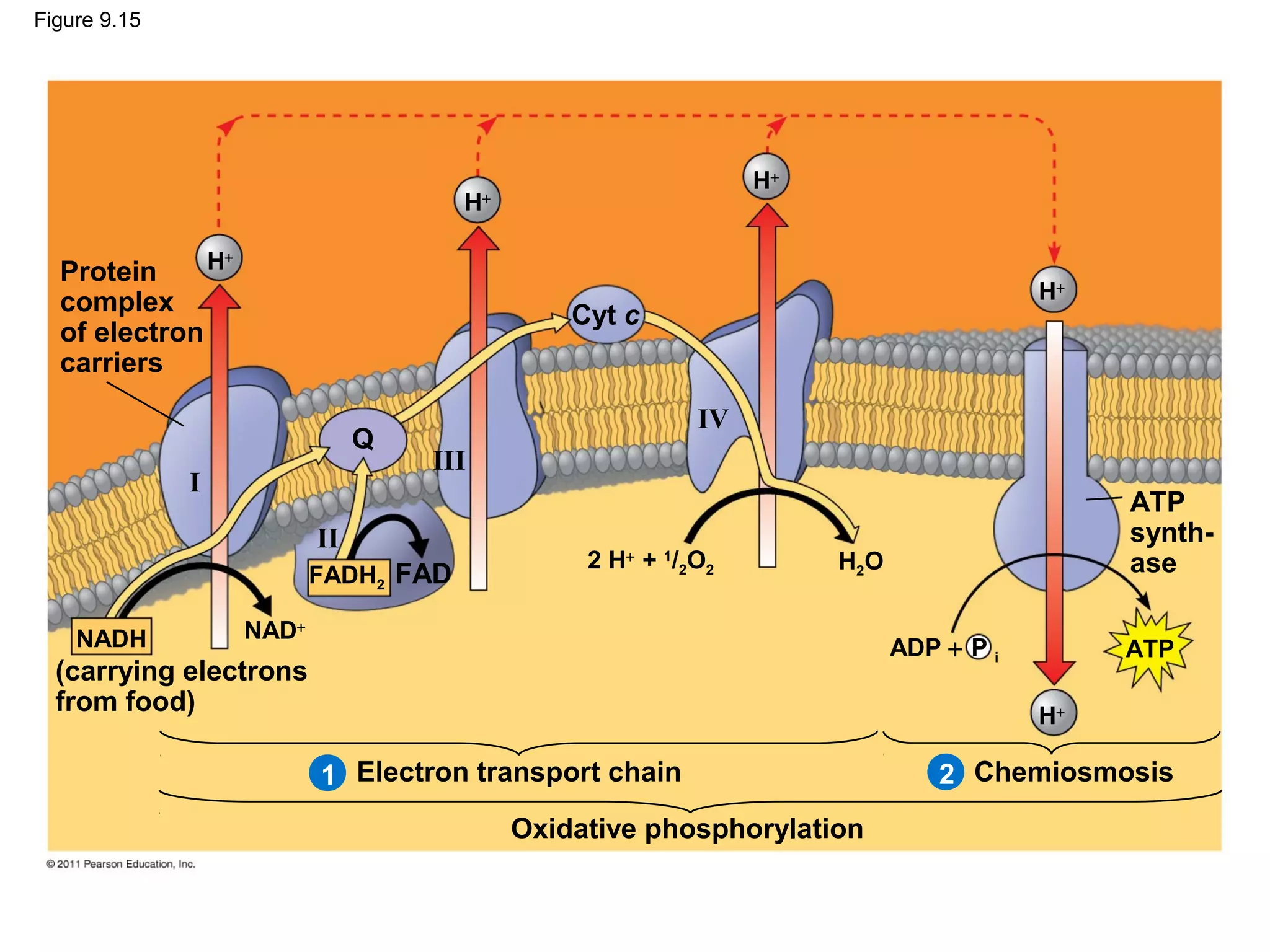
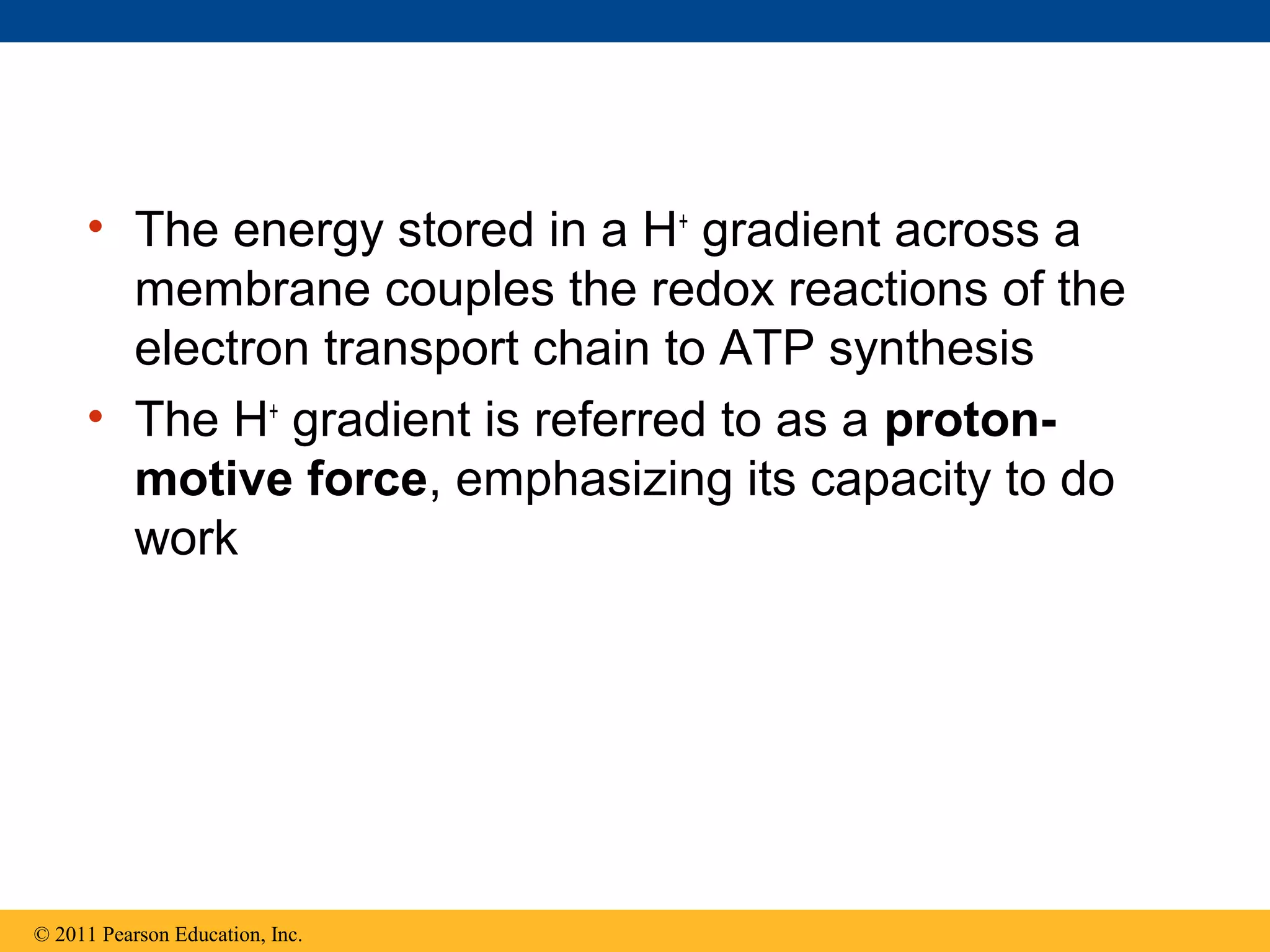
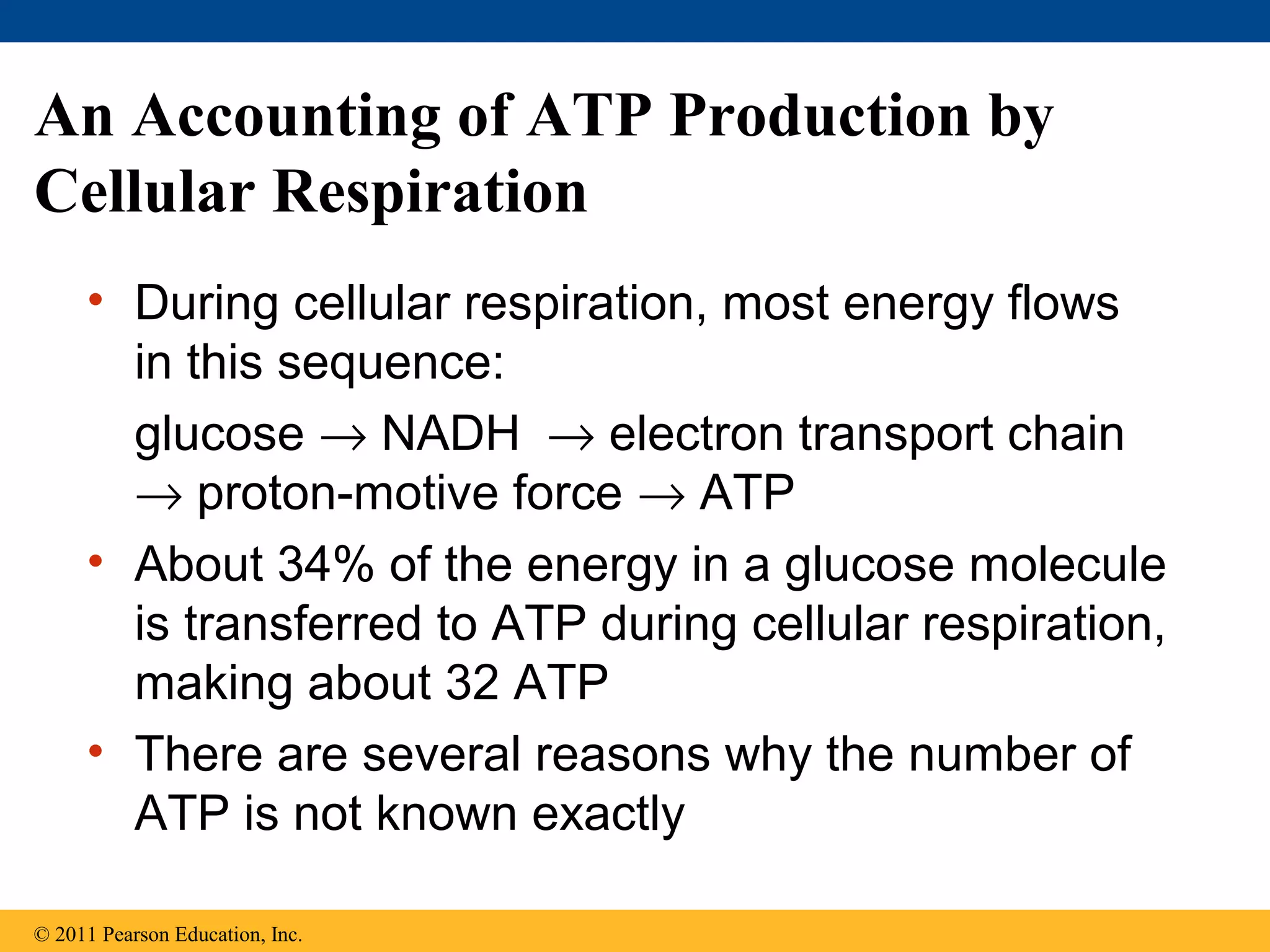
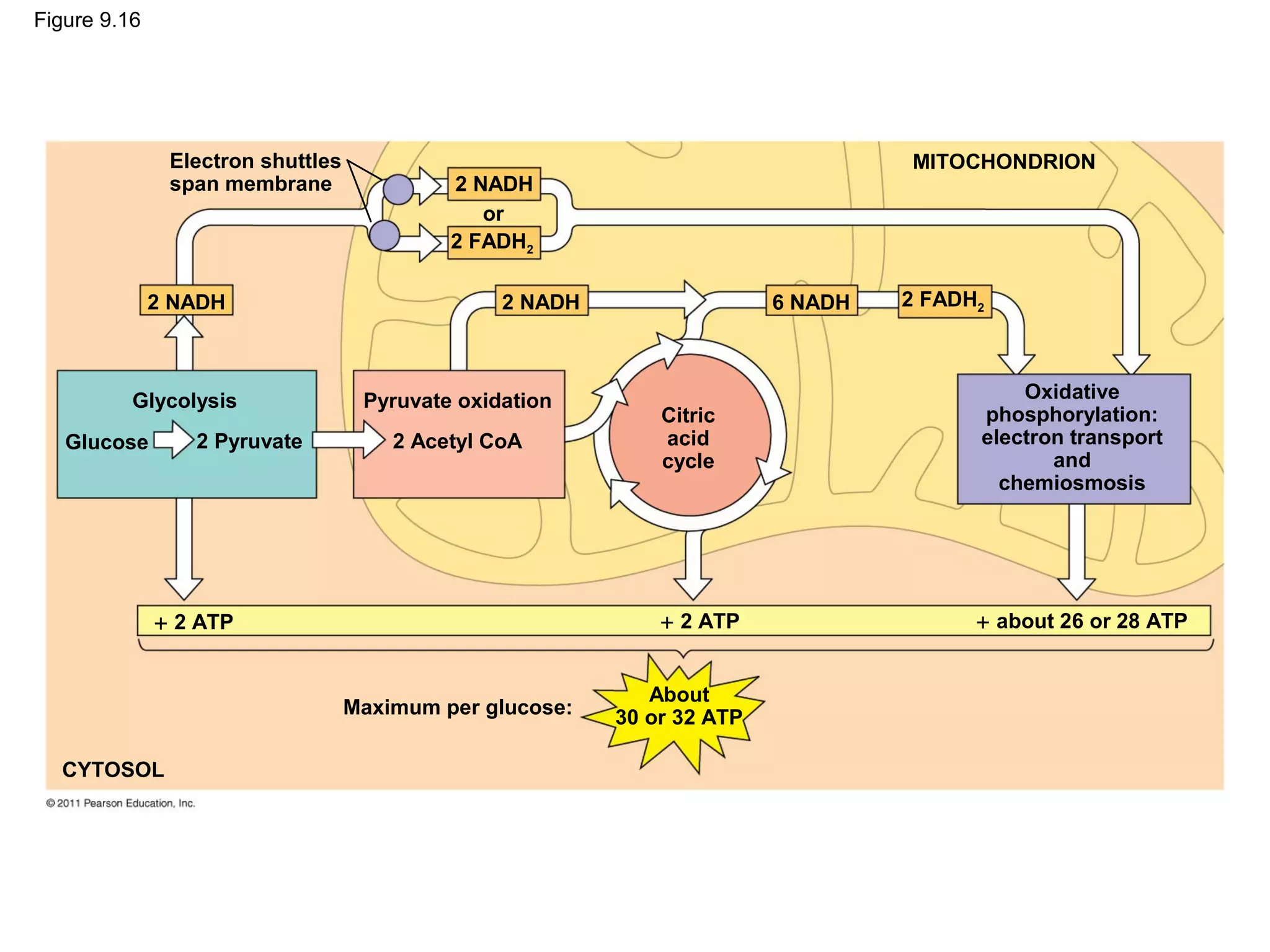
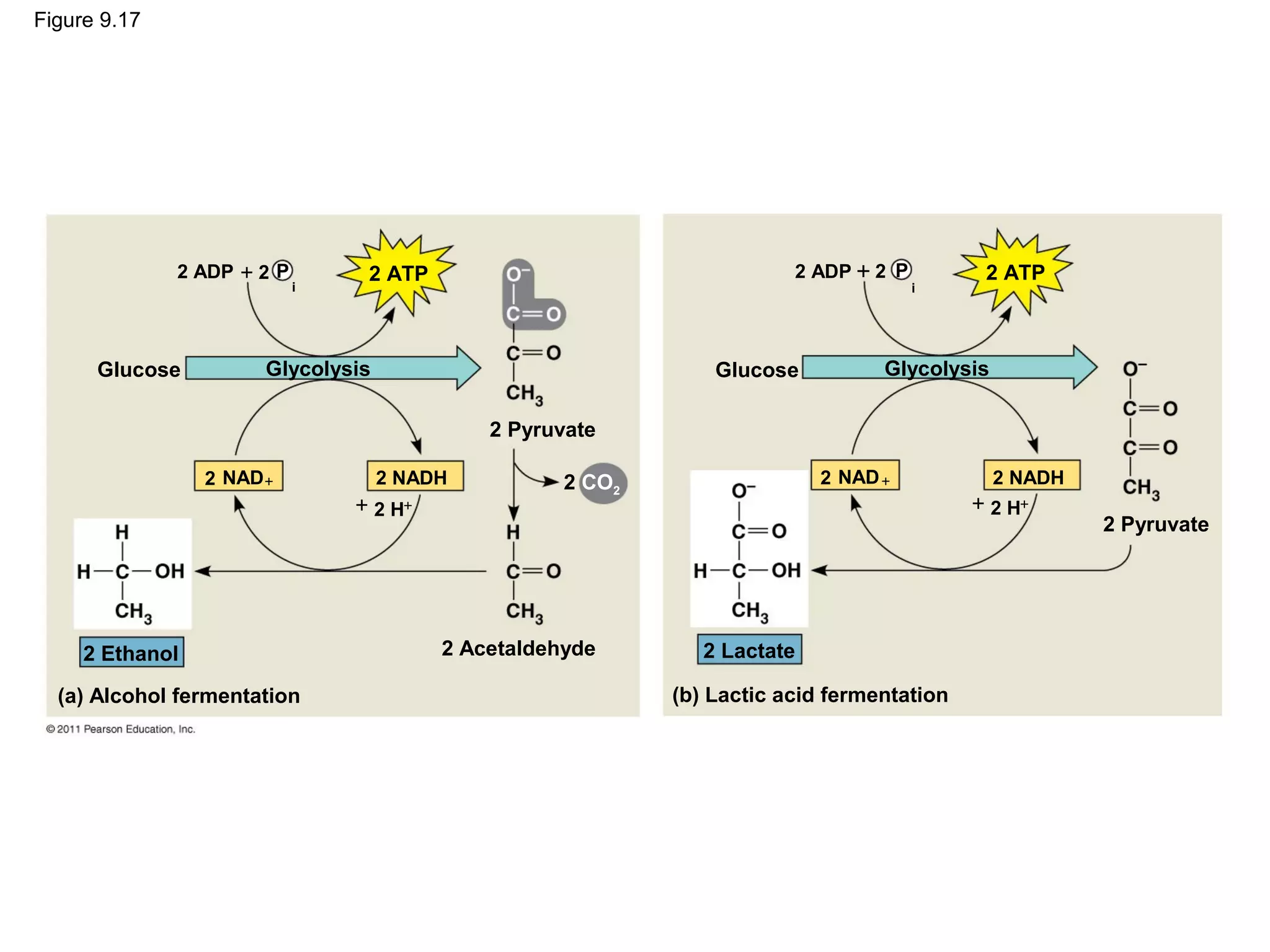
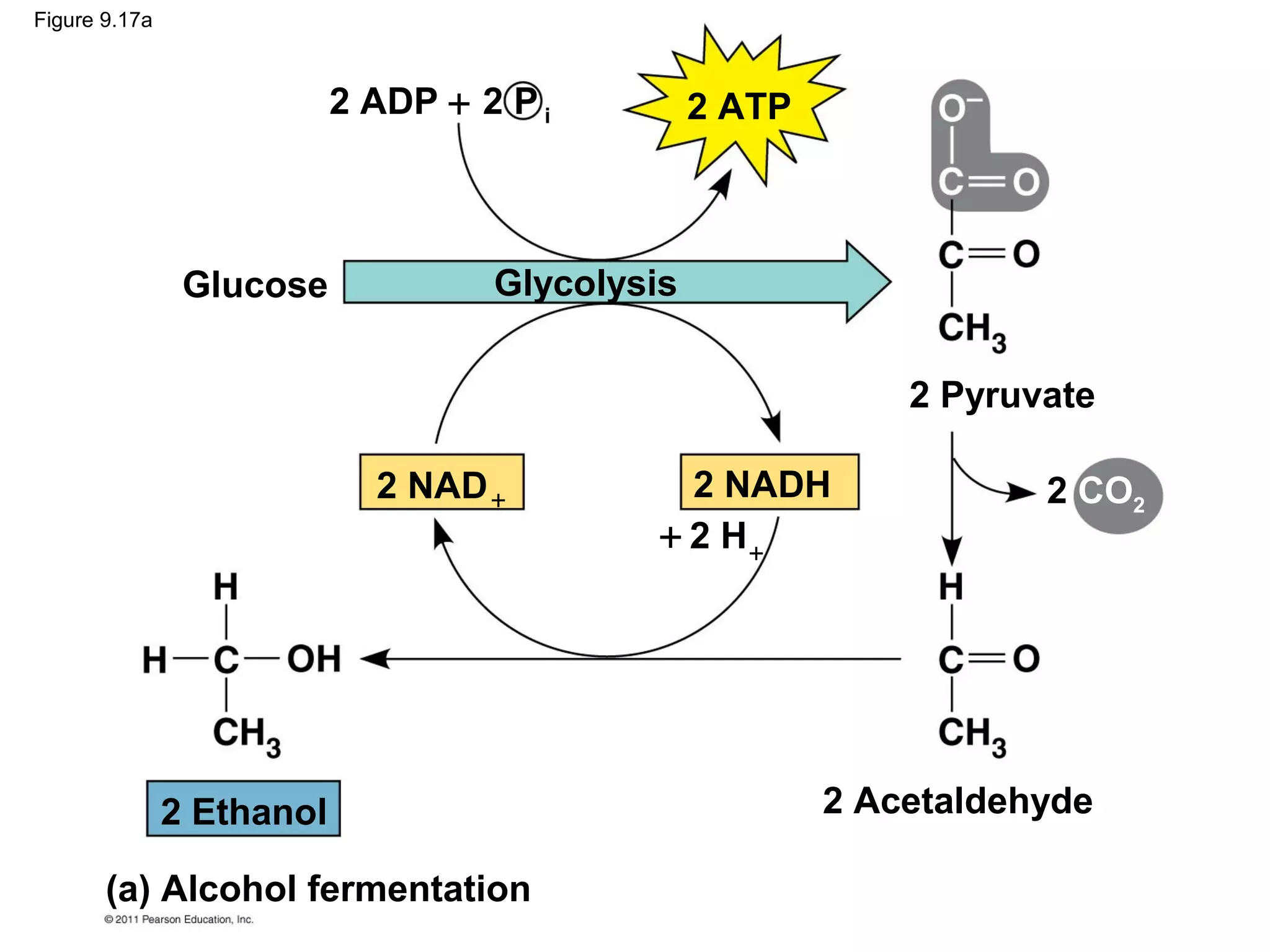
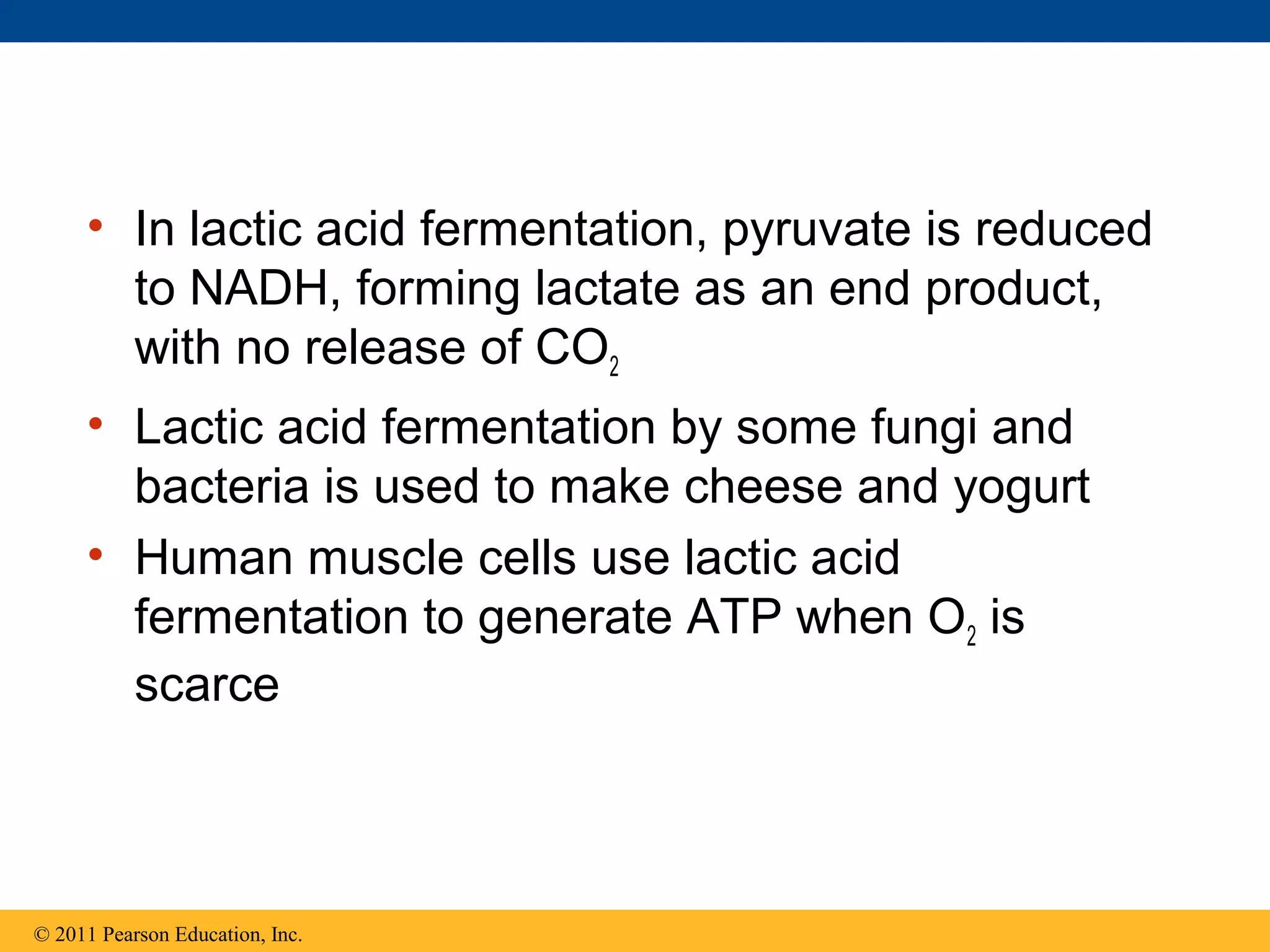
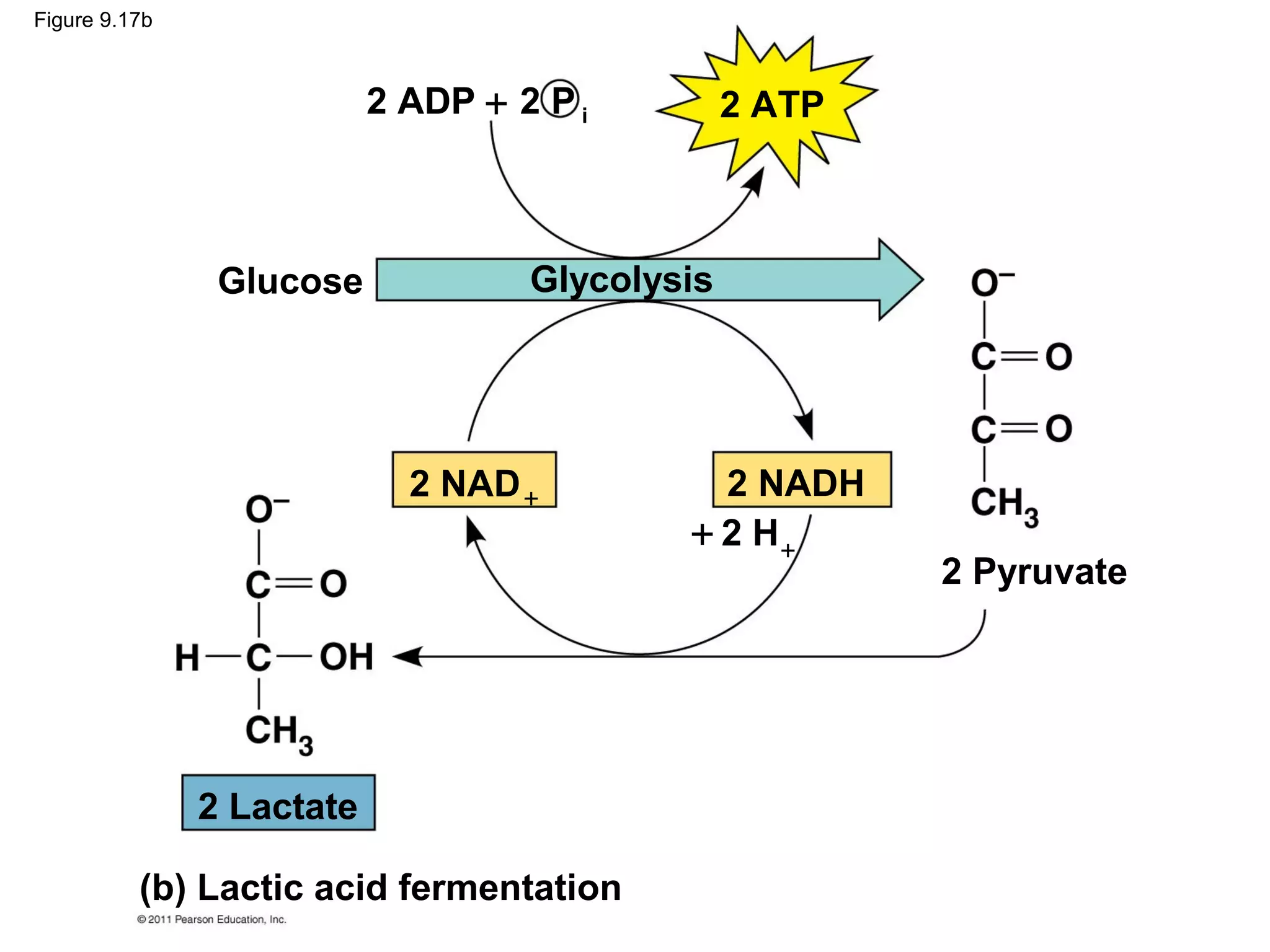
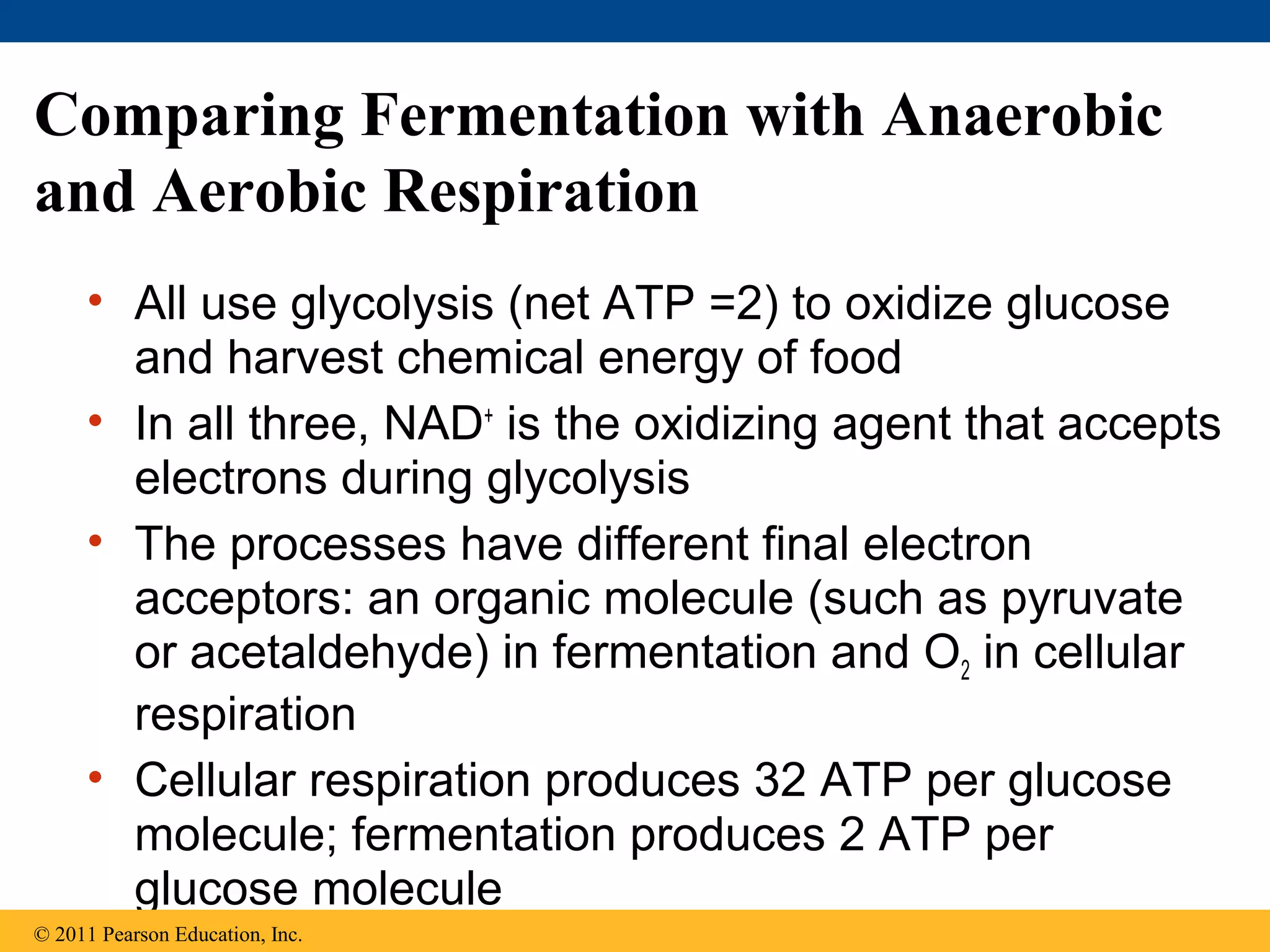
This document provides an overview and summaries of key concepts from Chapter 9 of Campbell Biology, Ninth Edition, which discusses cellular respiration and fermentation. The chapter examines how cells harvest energy from organic molecules like glucose through three main stages: glycolysis, the citric acid cycle, and oxidative phosphorylation. Glycolysis breaks down glucose into pyruvate, generating a small amount of ATP. The citric acid cycle then completes the oxidation of pyruvate. Finally, oxidative phosphorylation involves an electron transport chain that generates the majority of ATP.