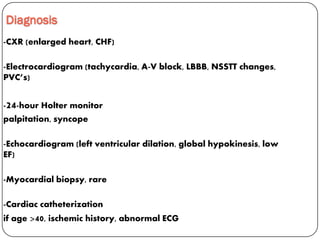
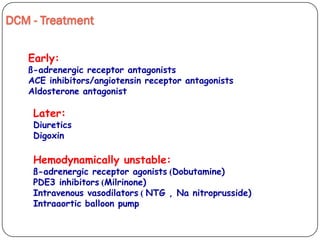
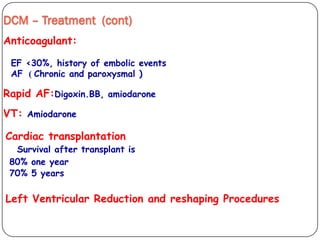
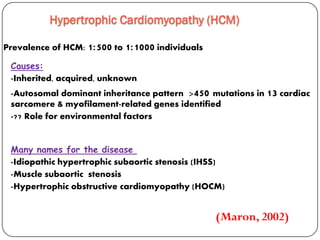
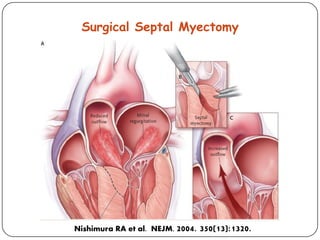
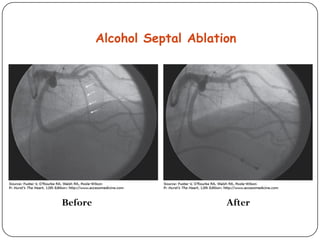
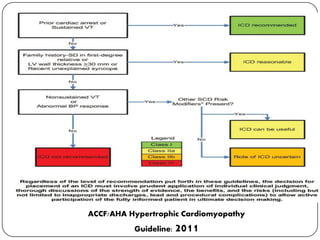
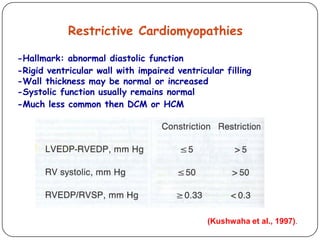
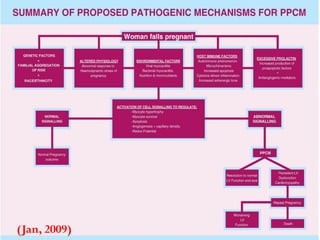
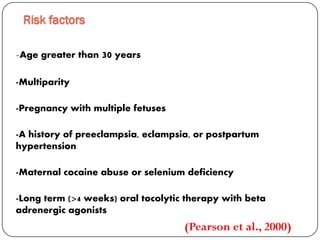
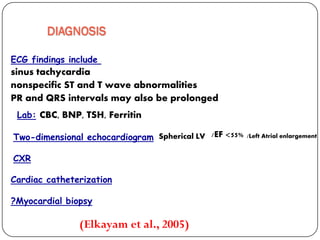
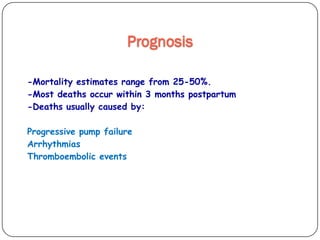
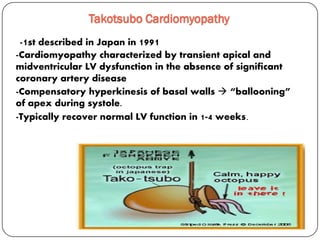
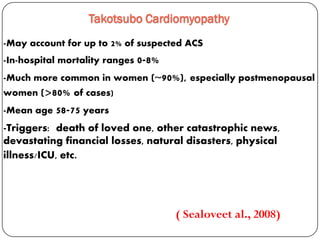
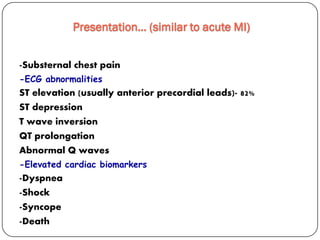
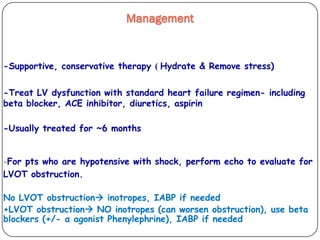
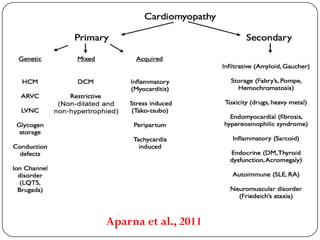
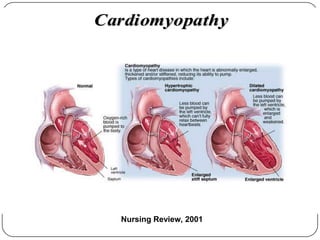
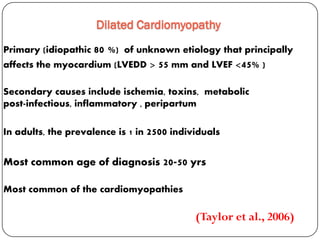
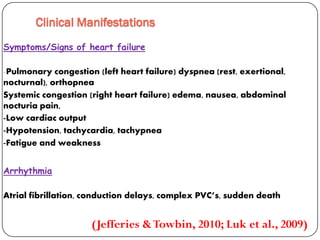
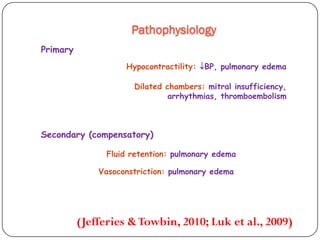
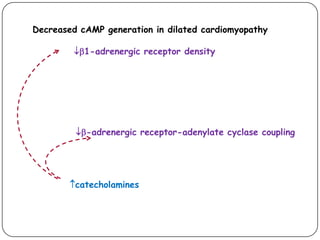
This document discusses various types of cardiomyopathy, including dilated cardiomyopathy, hypertrophic cardiomyopathy, restrictive cardiomyopathy, and Takotsubo cardiomyopathy. It covers the pathophysiology, clinical presentation, diagnosis, and management of each type. It also discusses prognosis and the use of stem cells in treating cardiomyopathy.


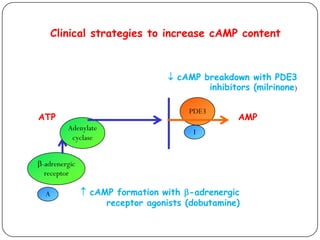
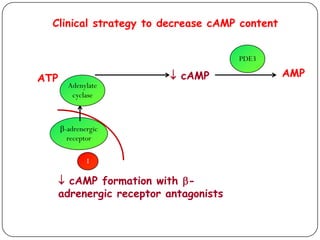
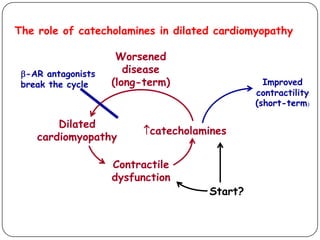
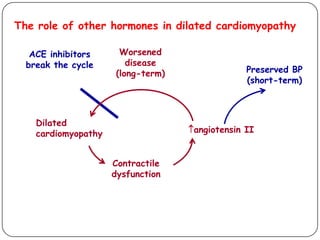
![Effect
on
[cAMP]
Short-term
effect
Long-term
effect
PDE3
inhibitors
contractility mortality
-adrenergic
receptor
agonists
contractility mortality
-adrenergic
receptor
antagonists
contractility mortality
cAMP-directed therapy: clinical results](https://image.slidesharecdn.com/cardiomyopathy3-180220163000/85/Cardiomyopathy-3-14-320.jpg)