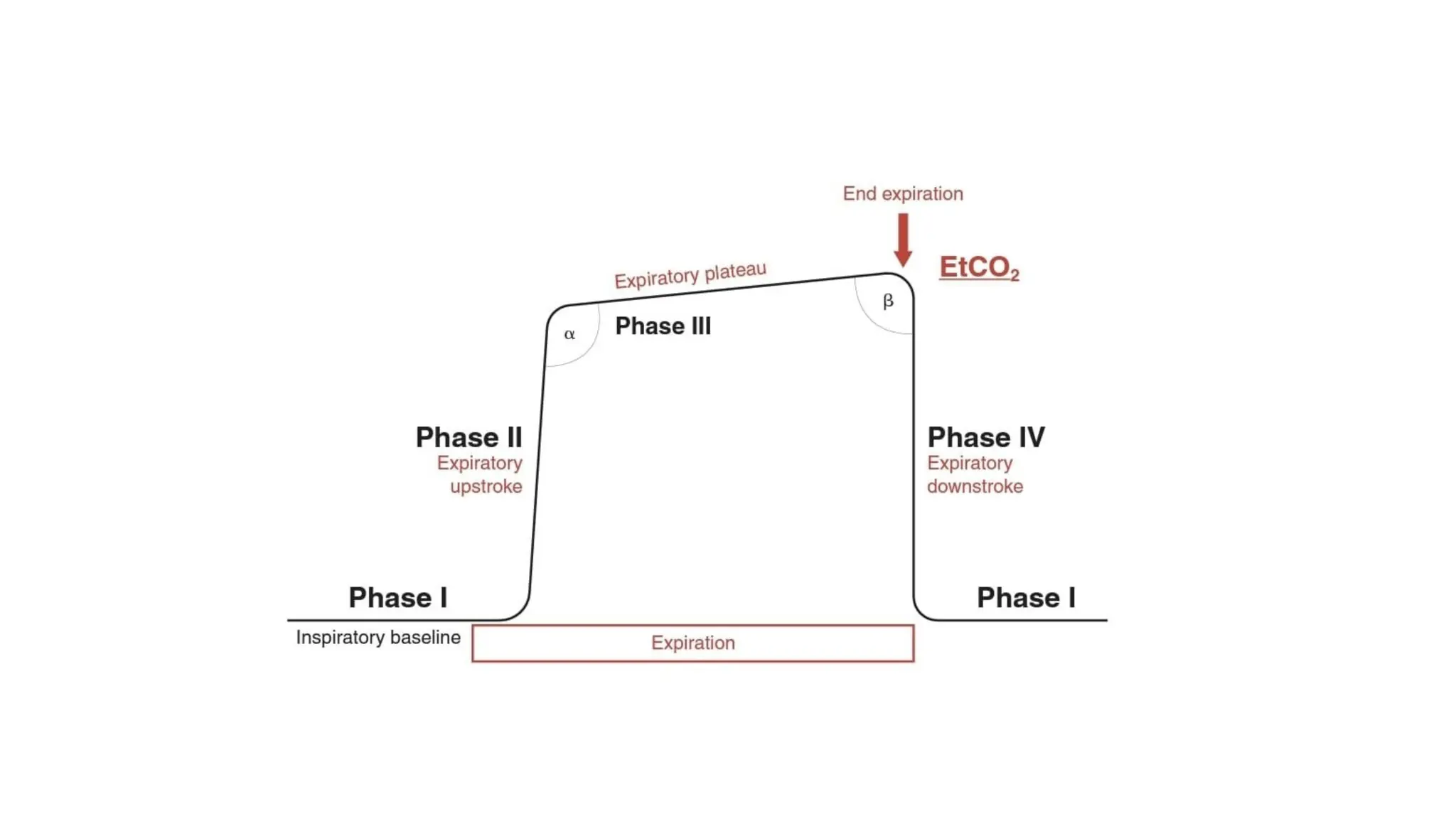
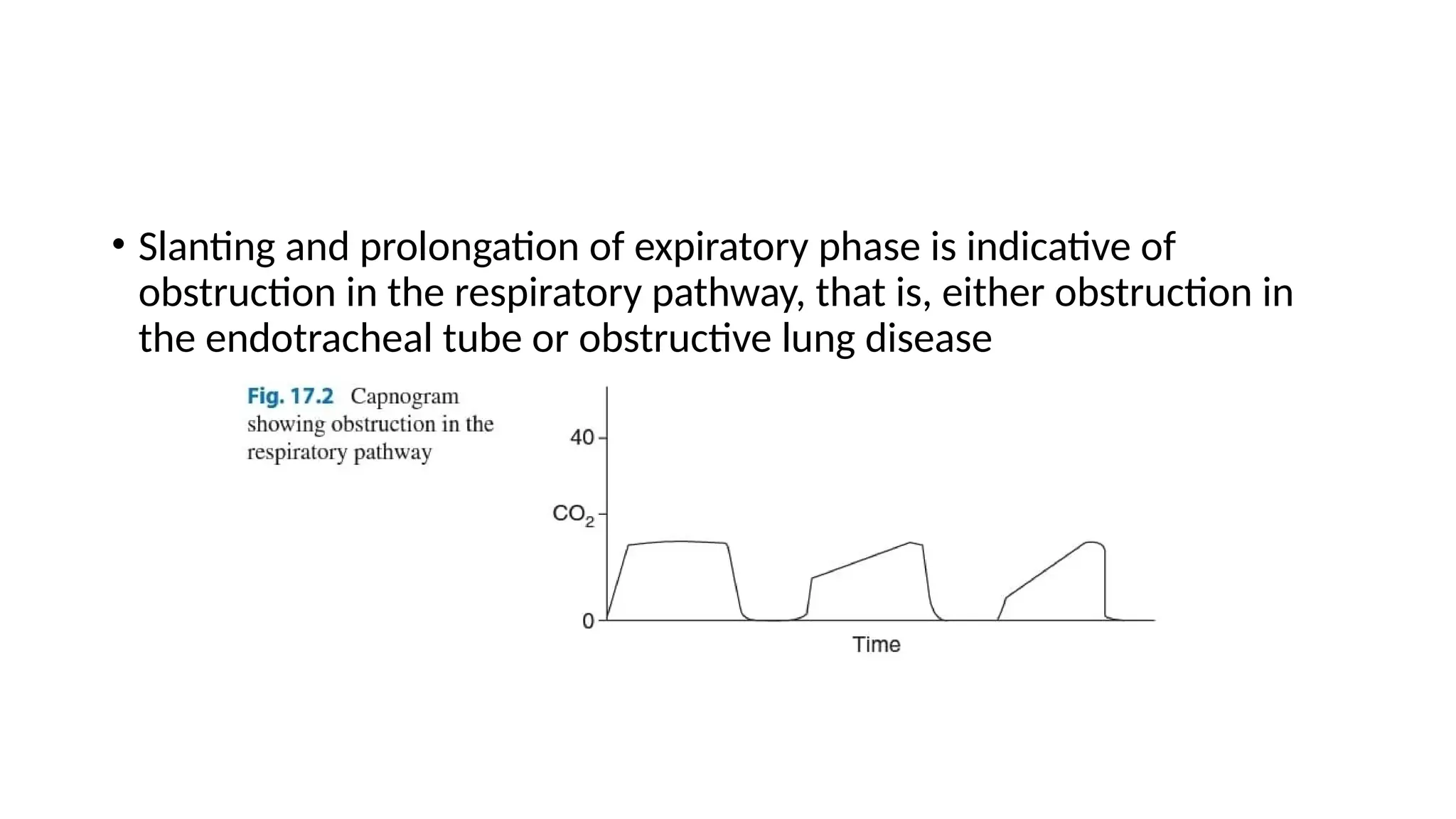
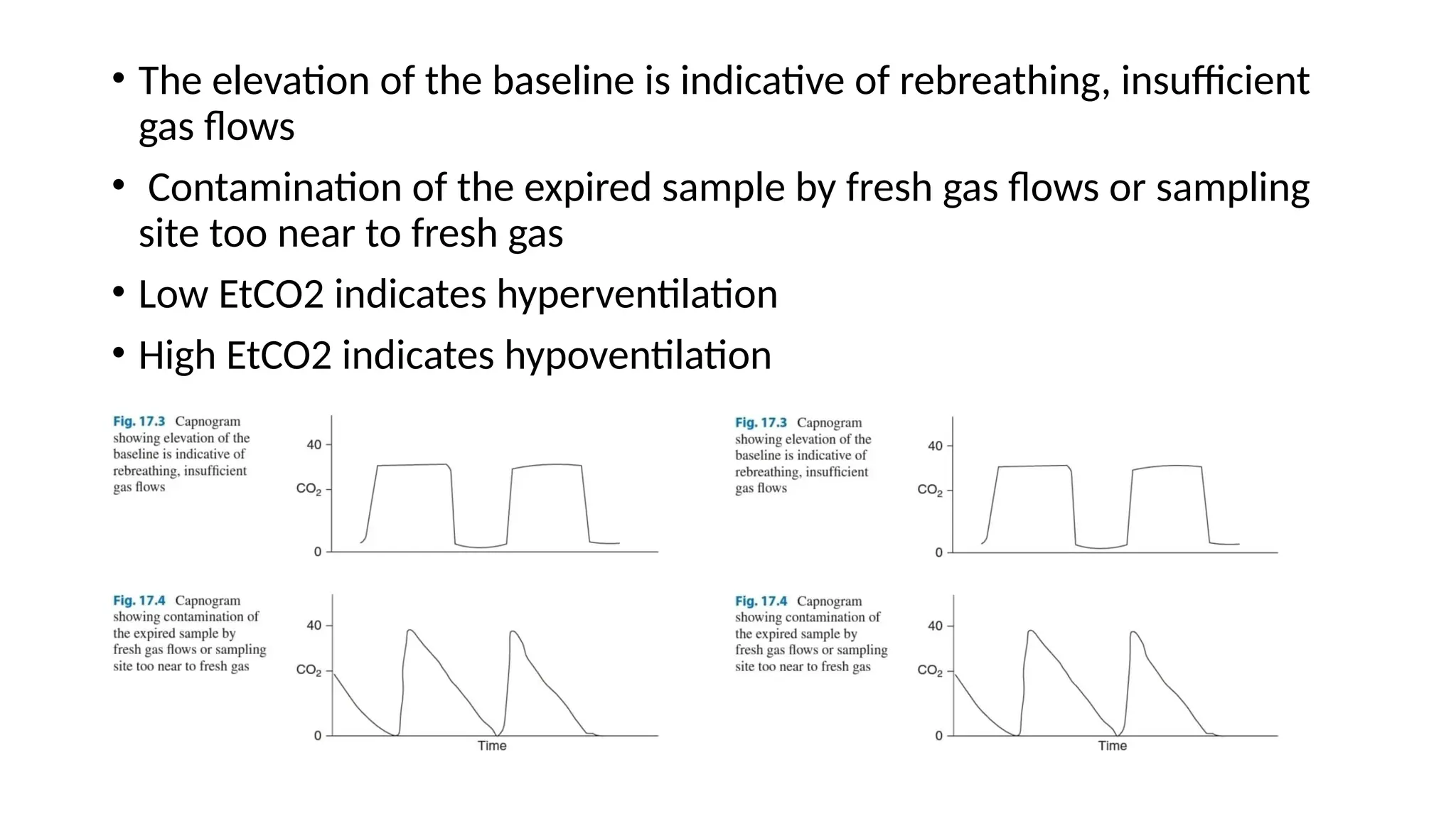
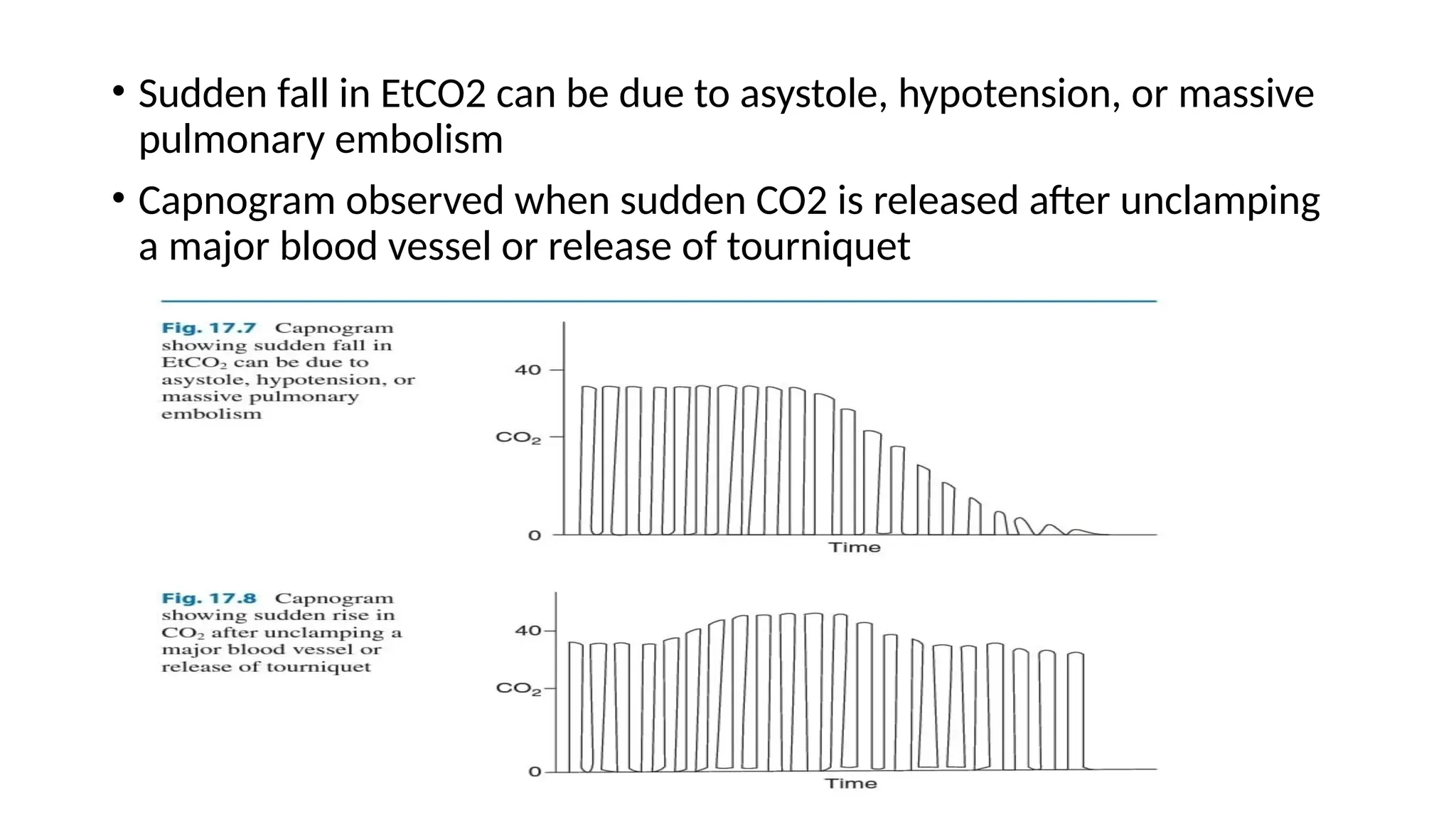
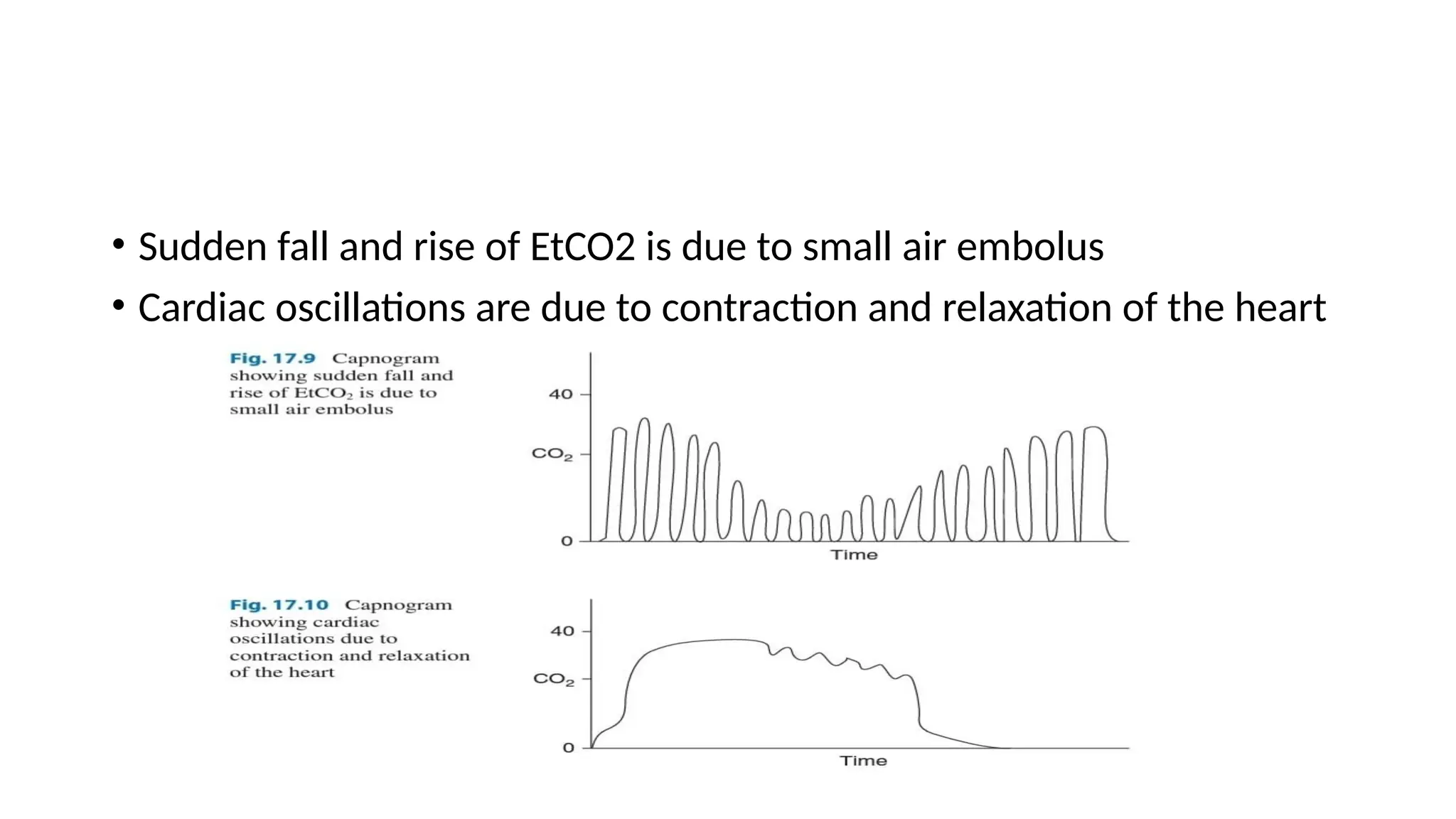
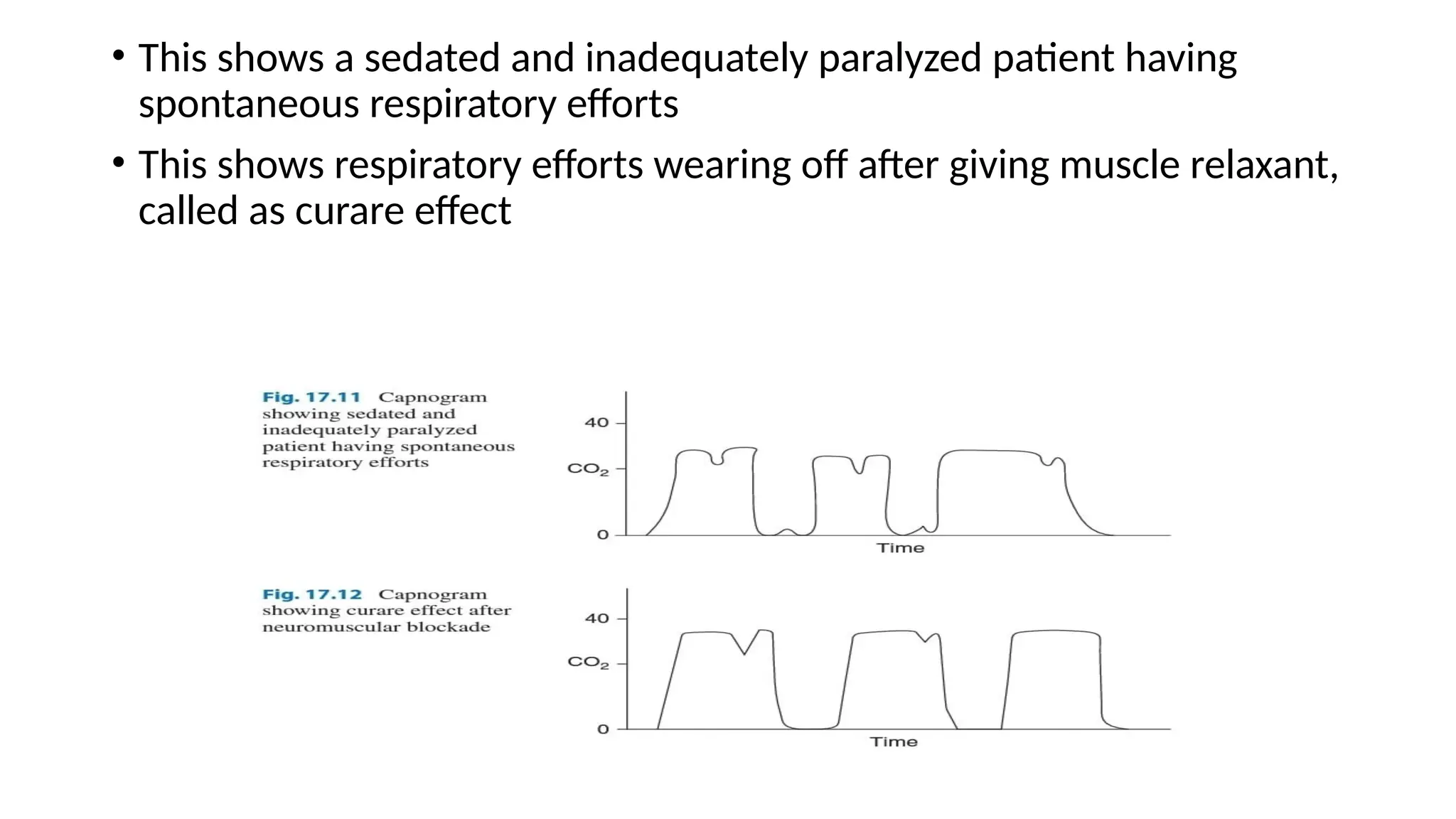
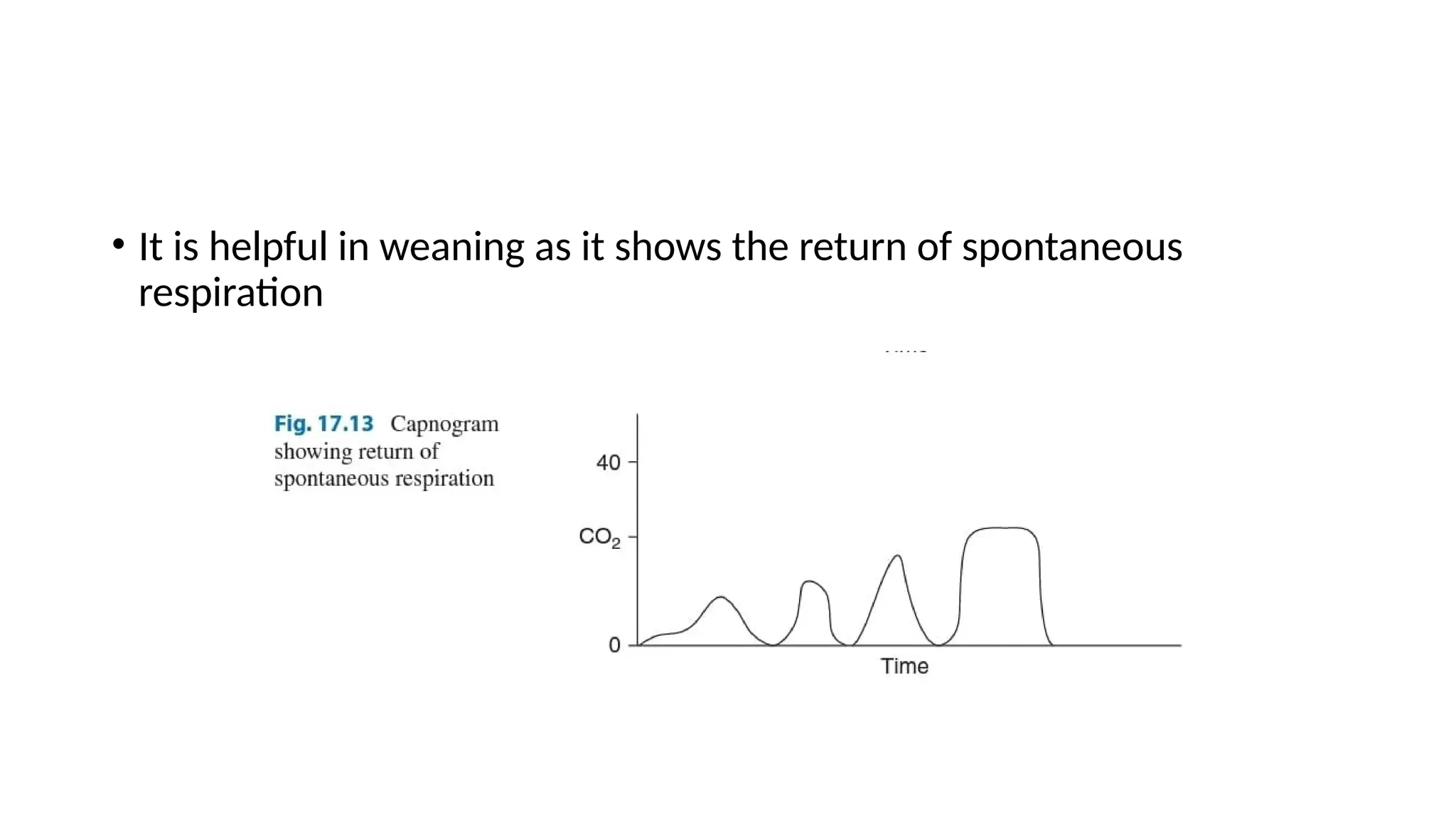
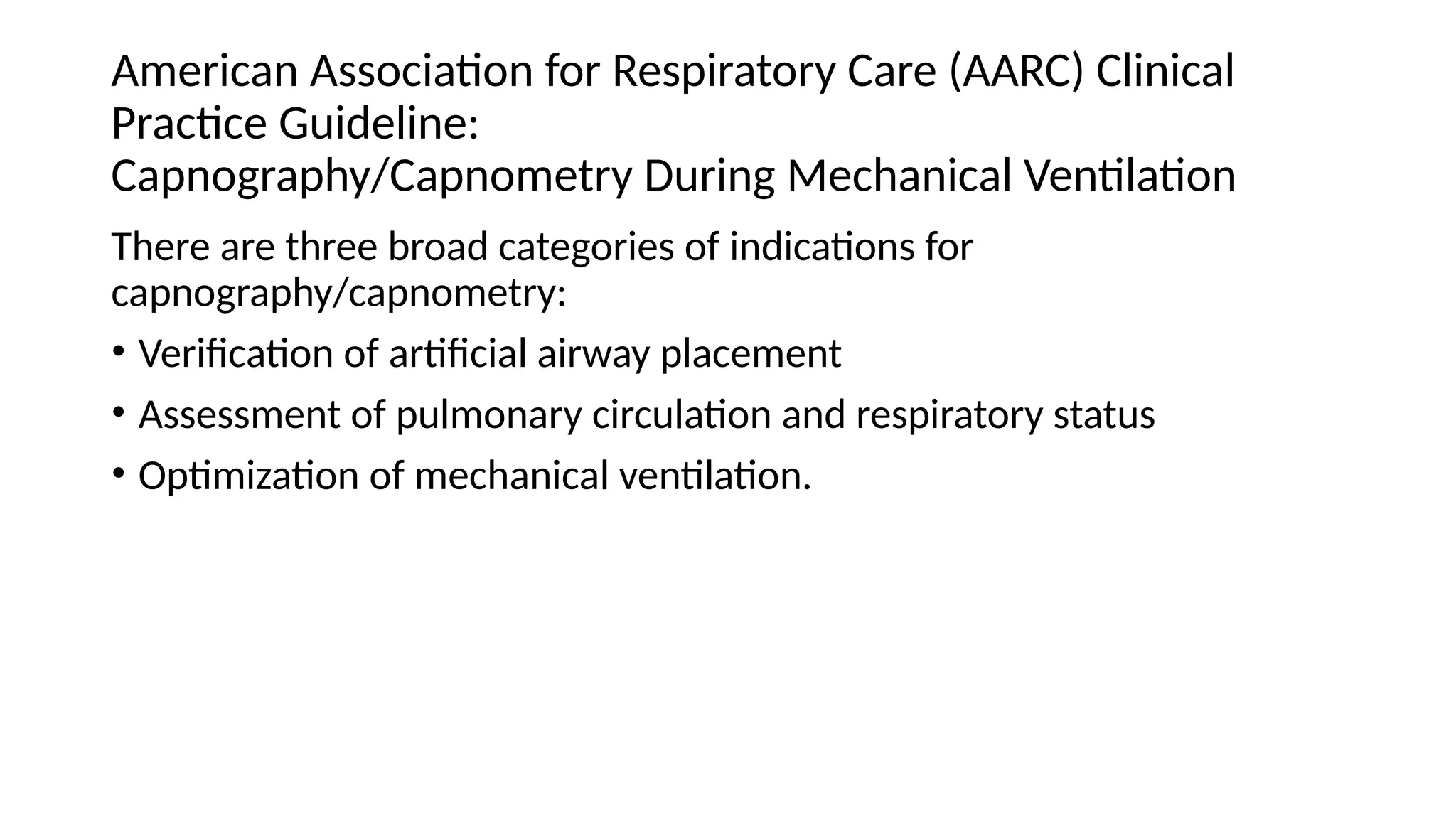
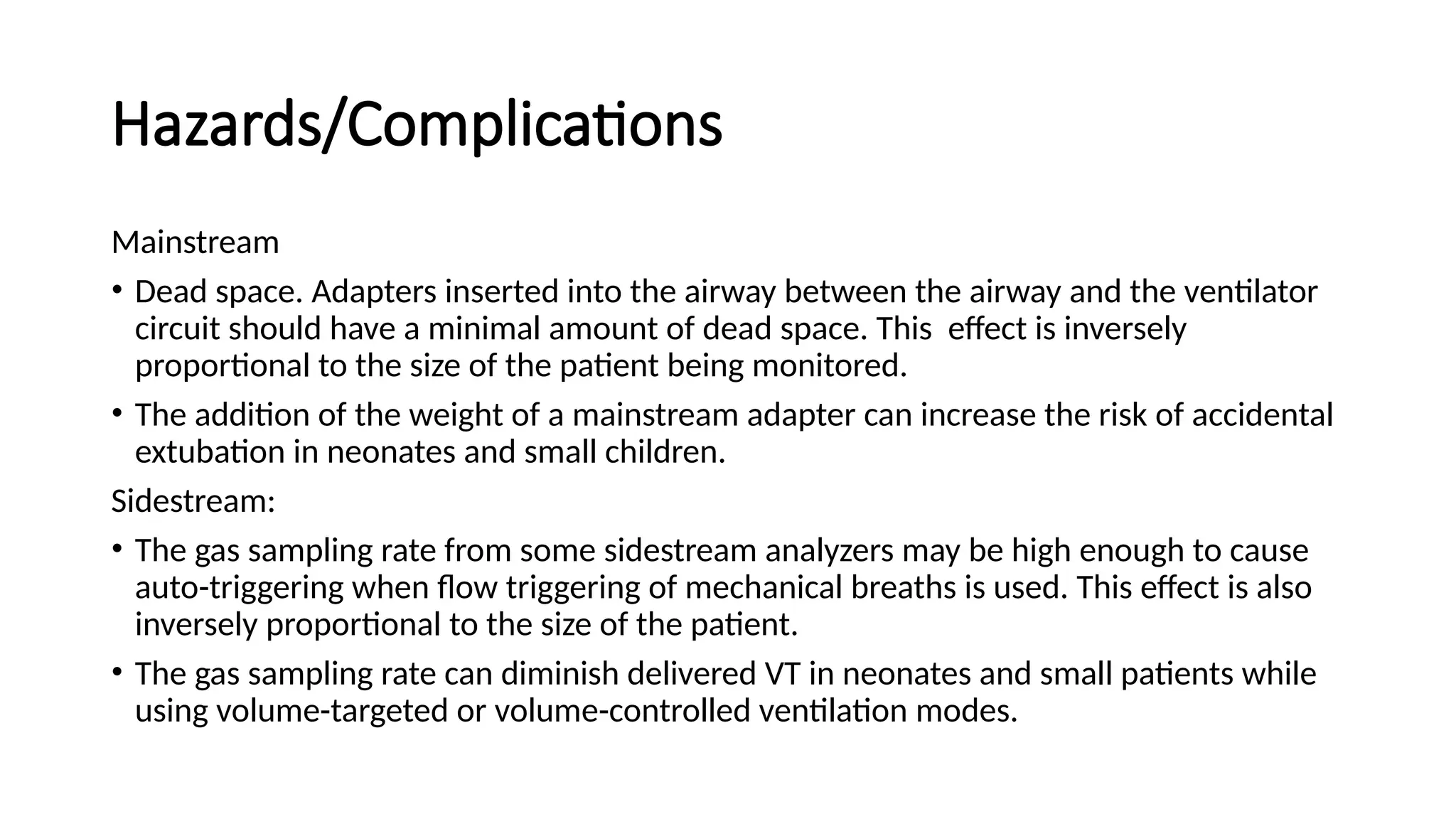
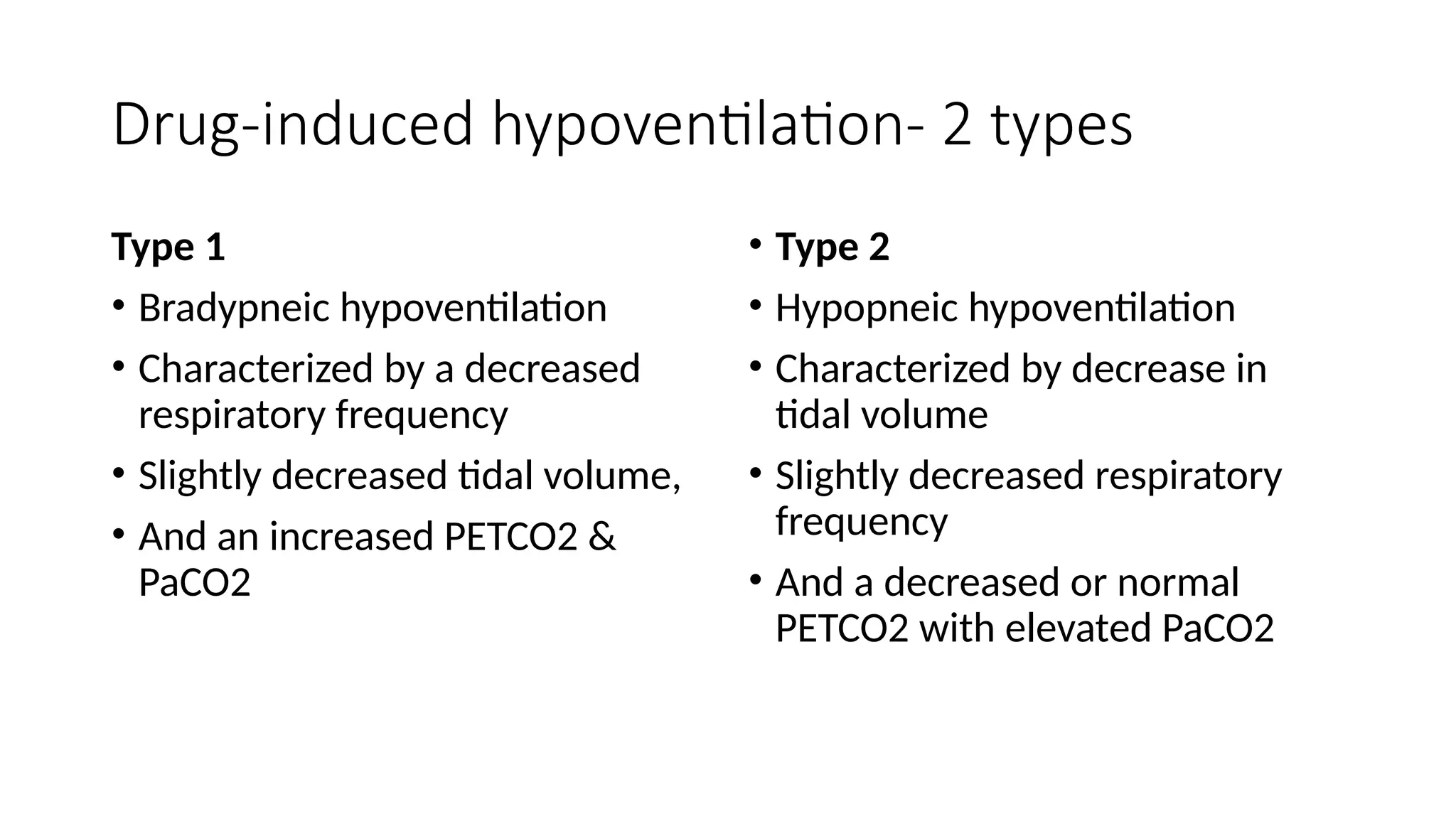
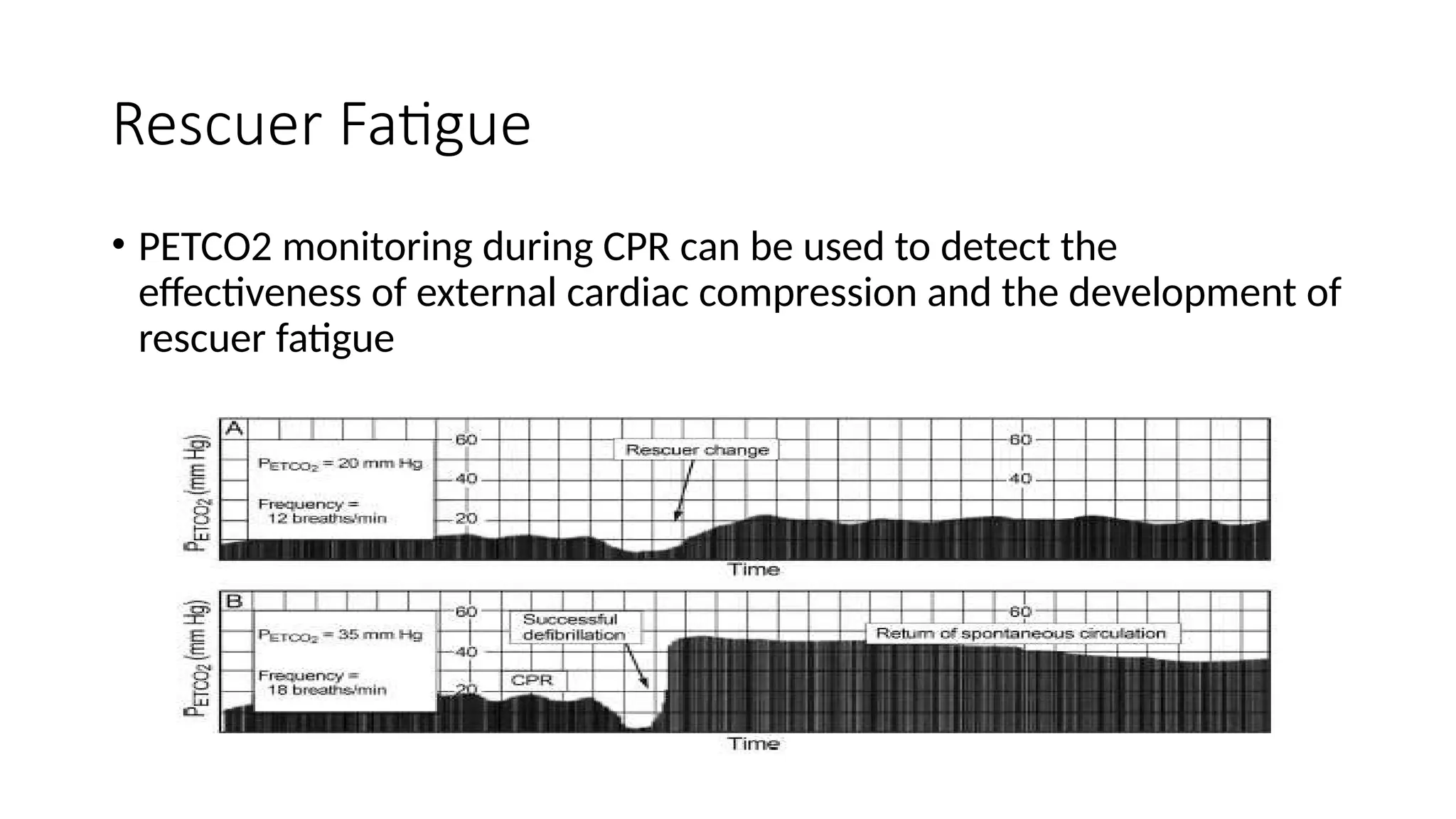
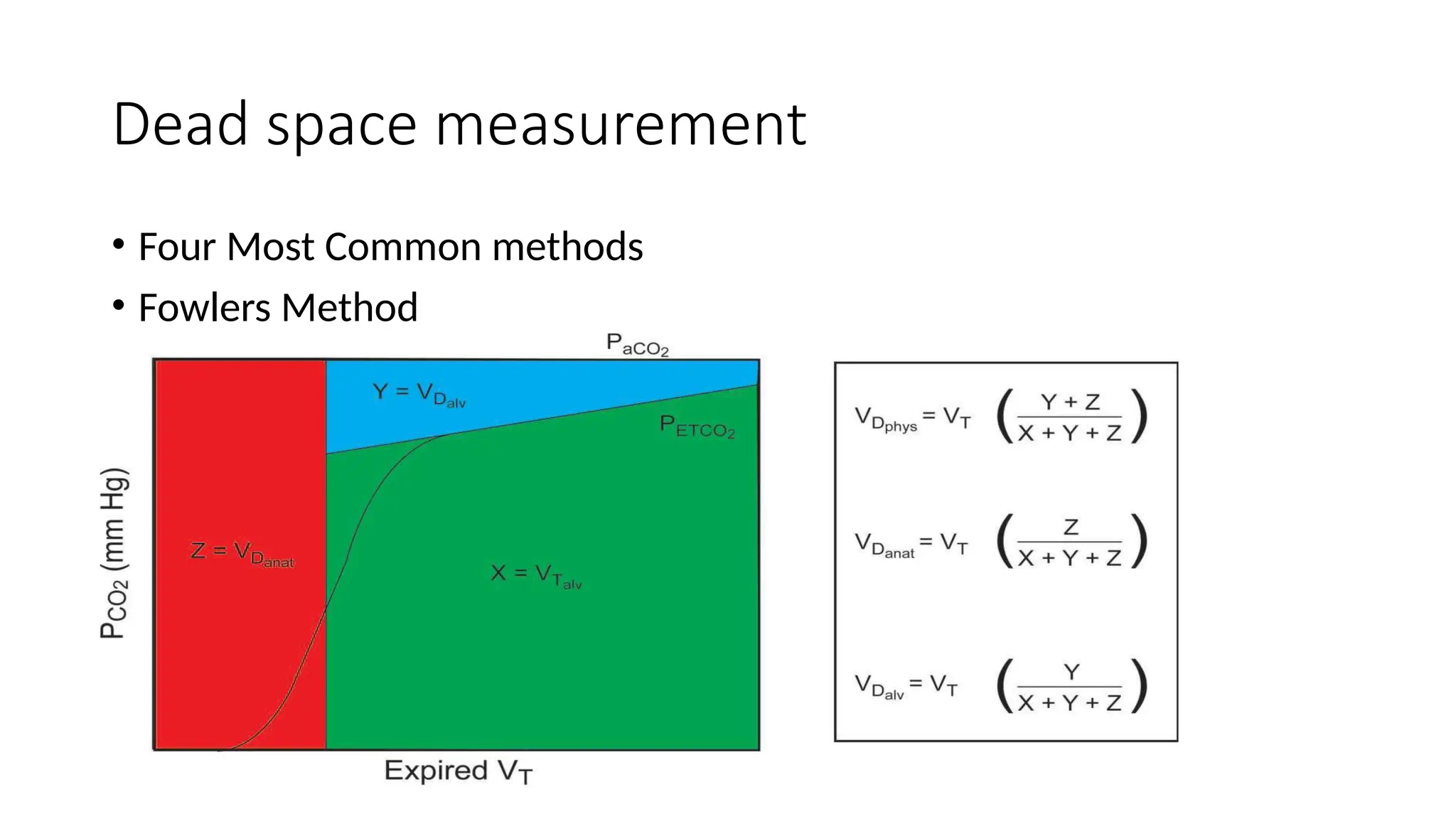
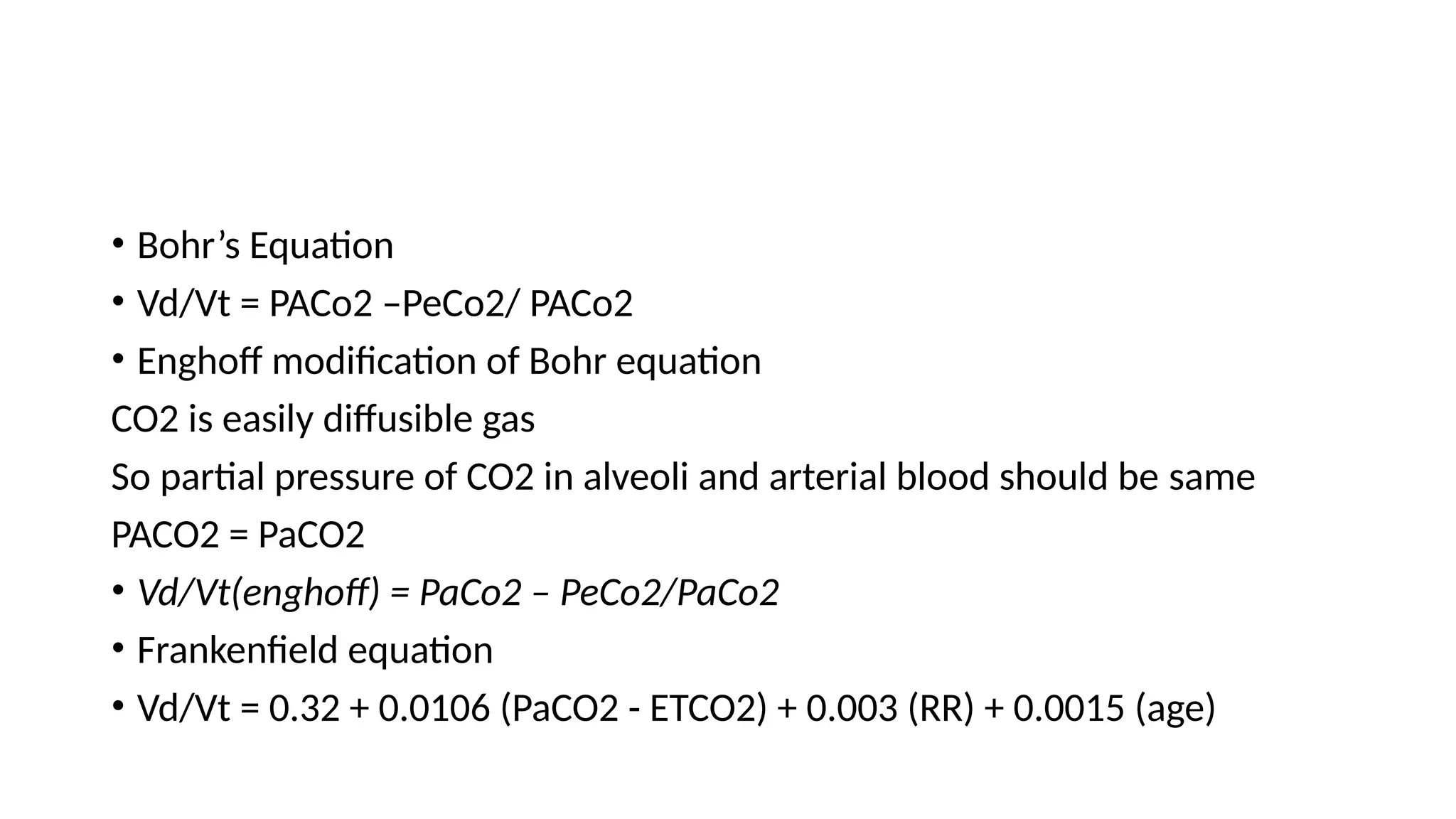
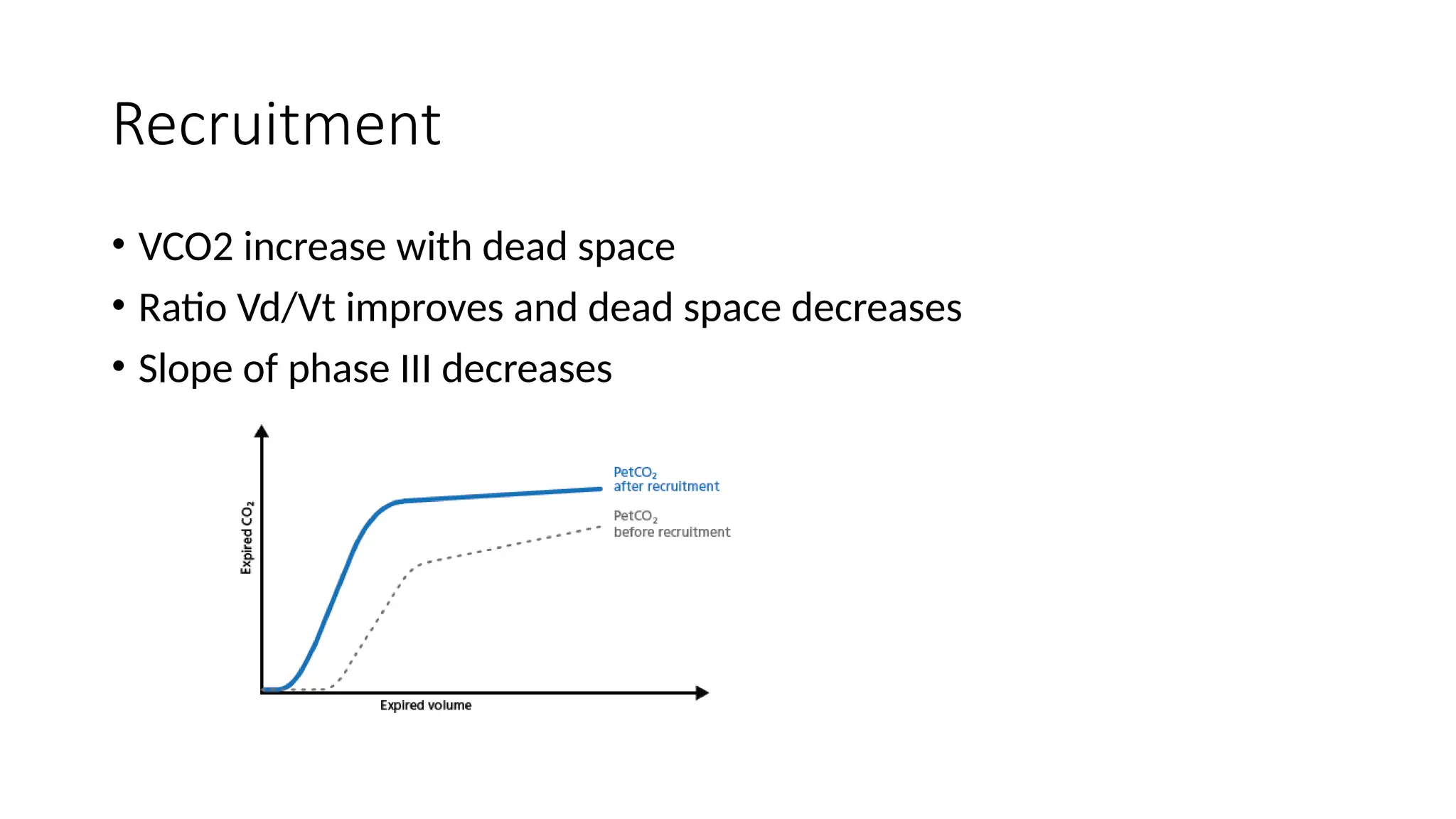
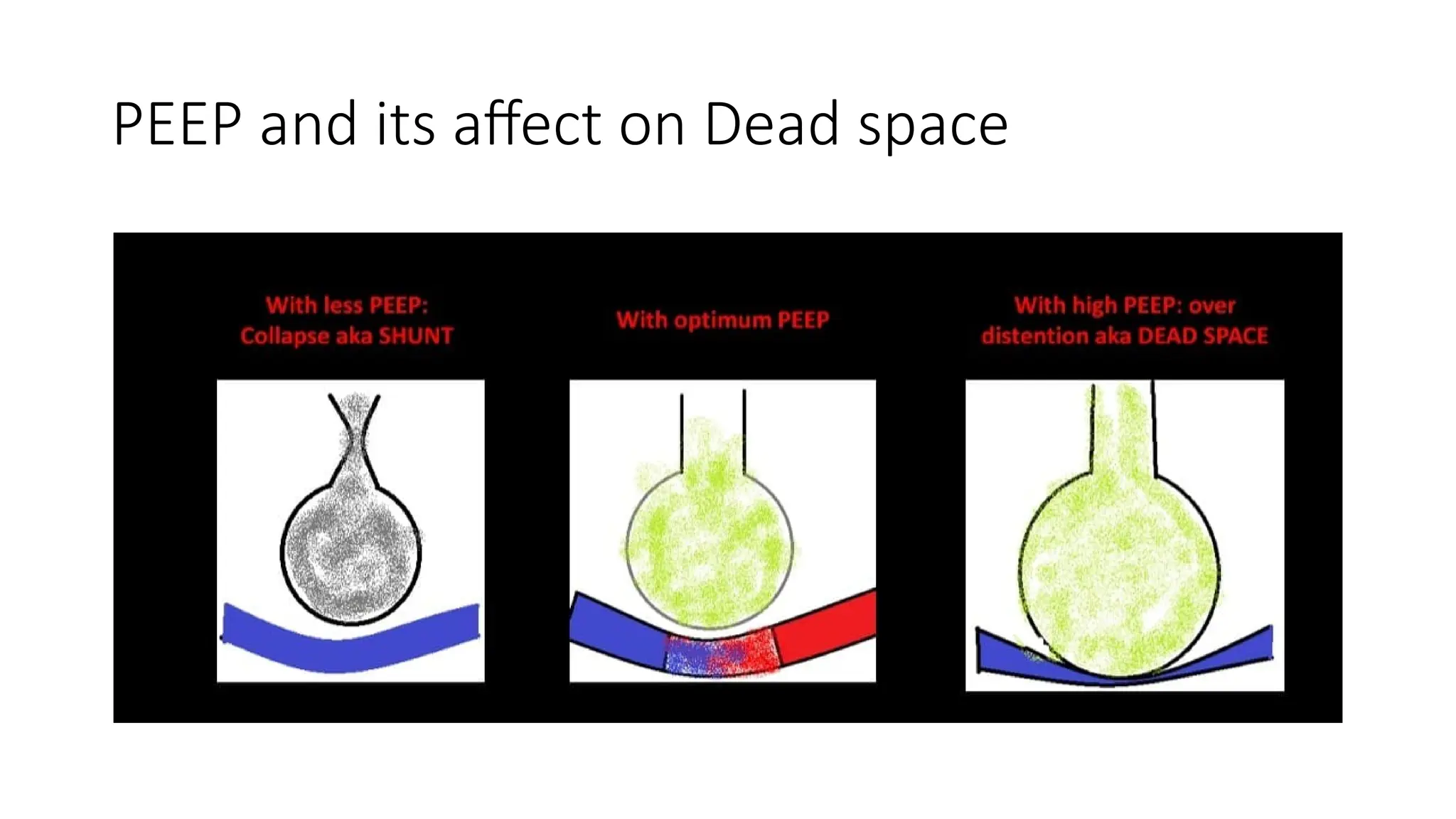
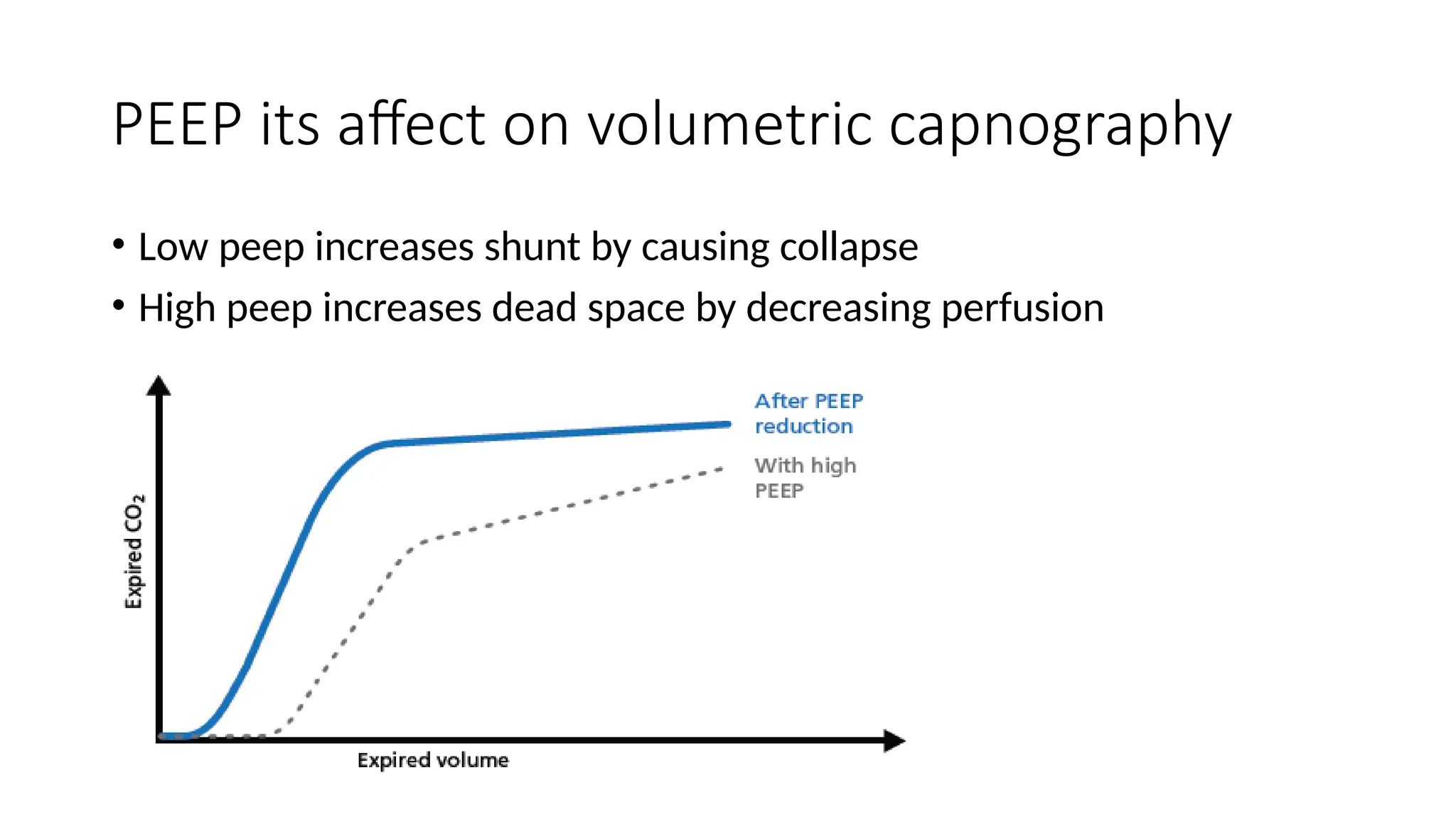
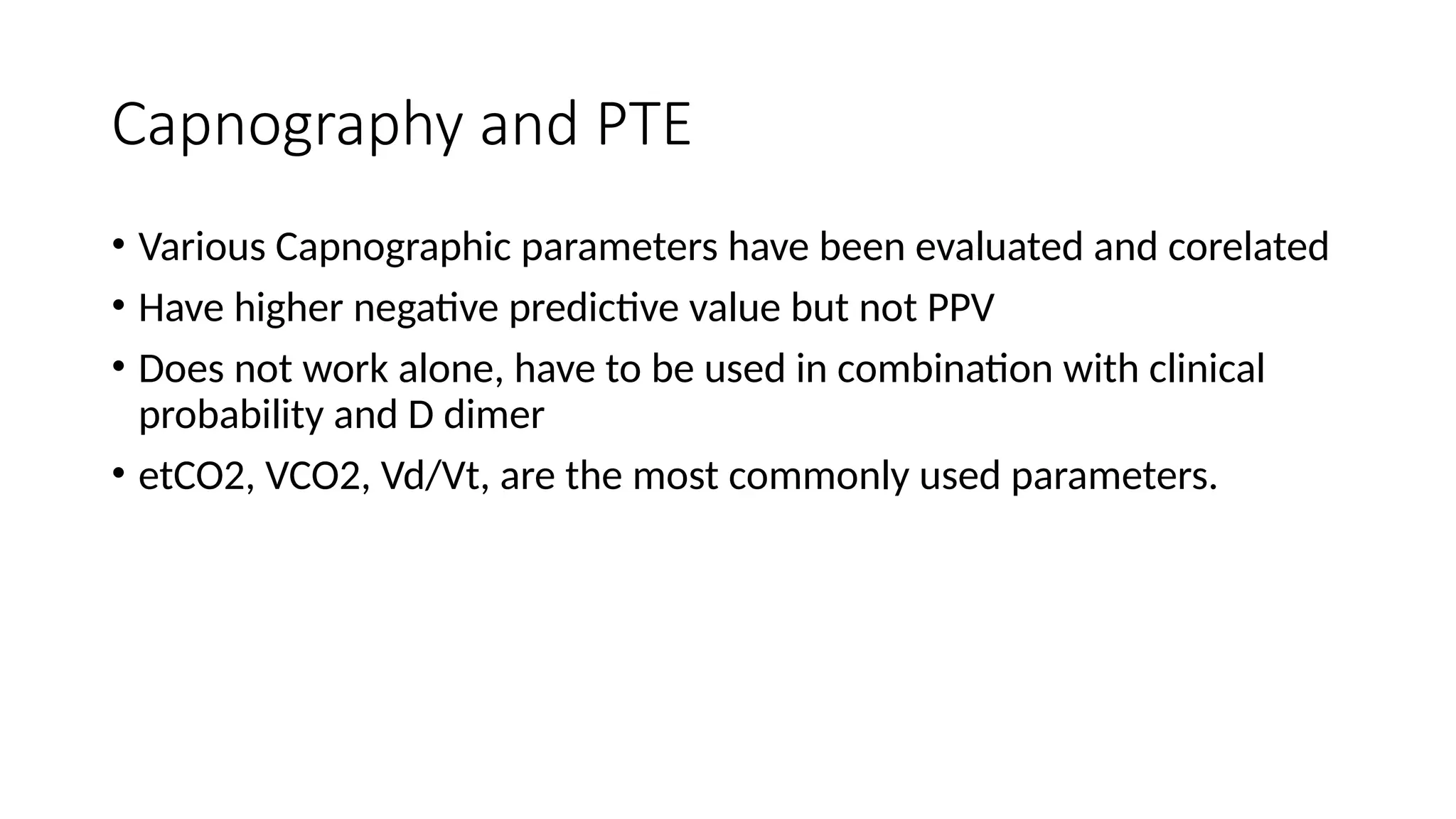
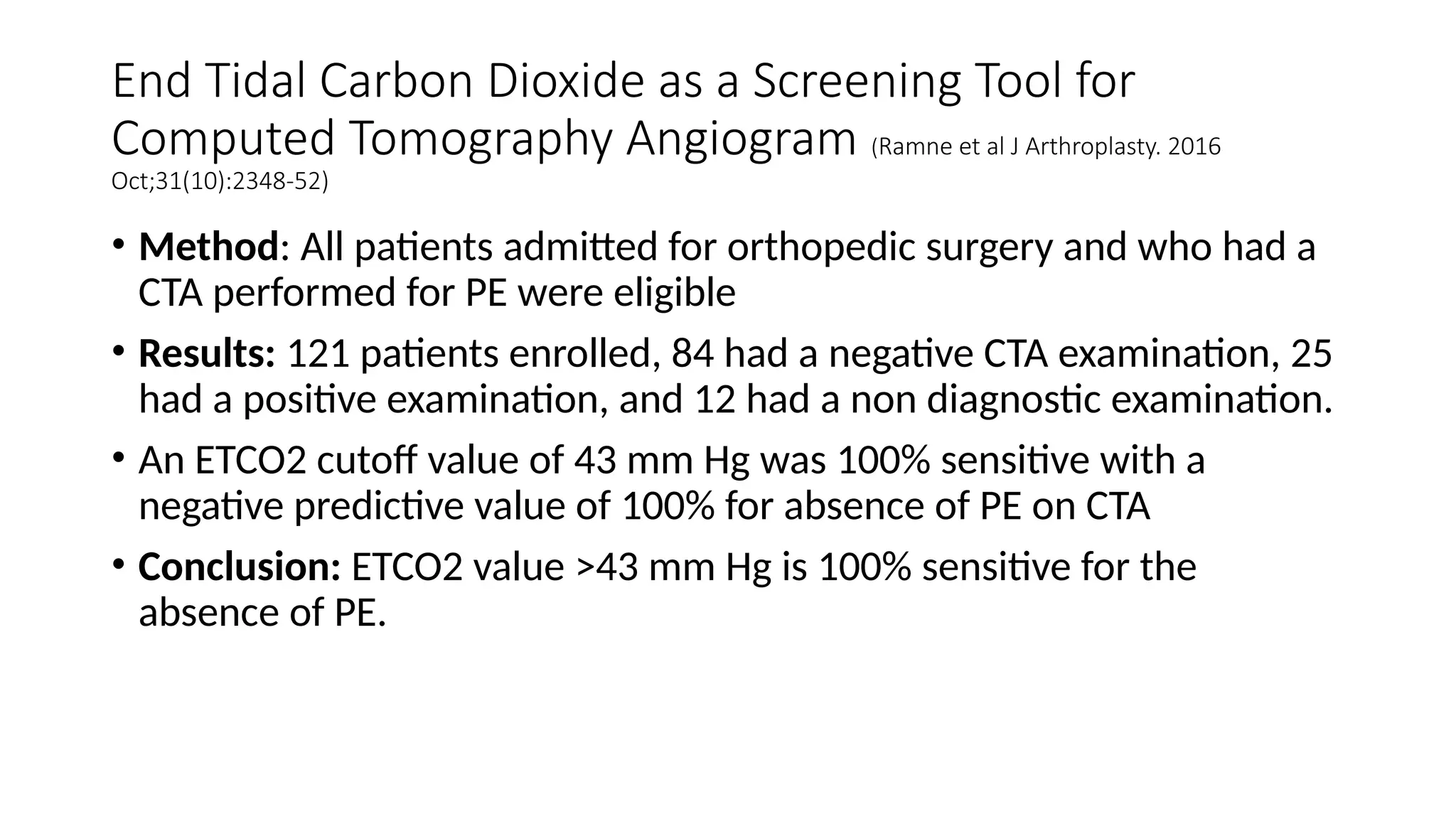
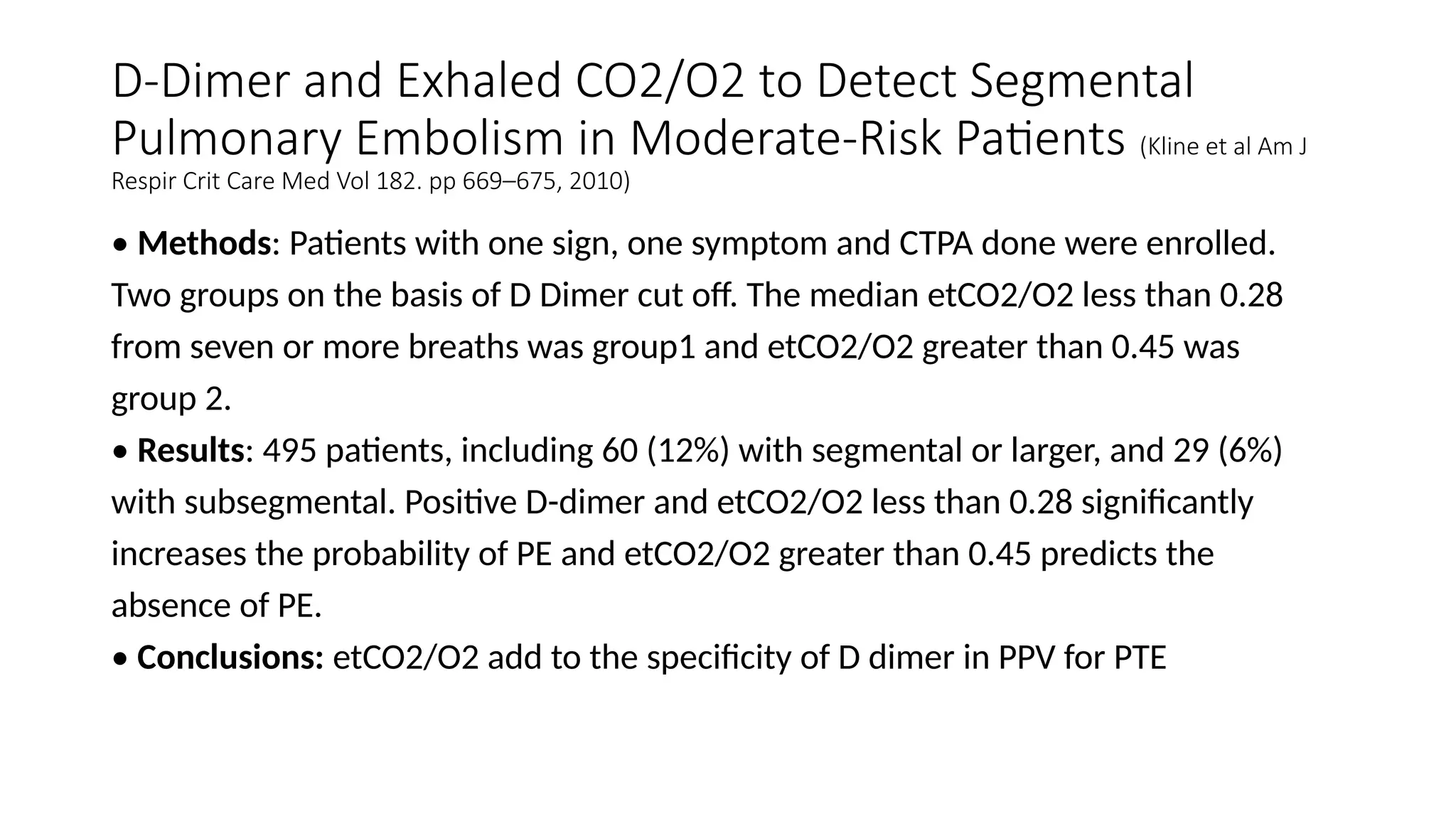
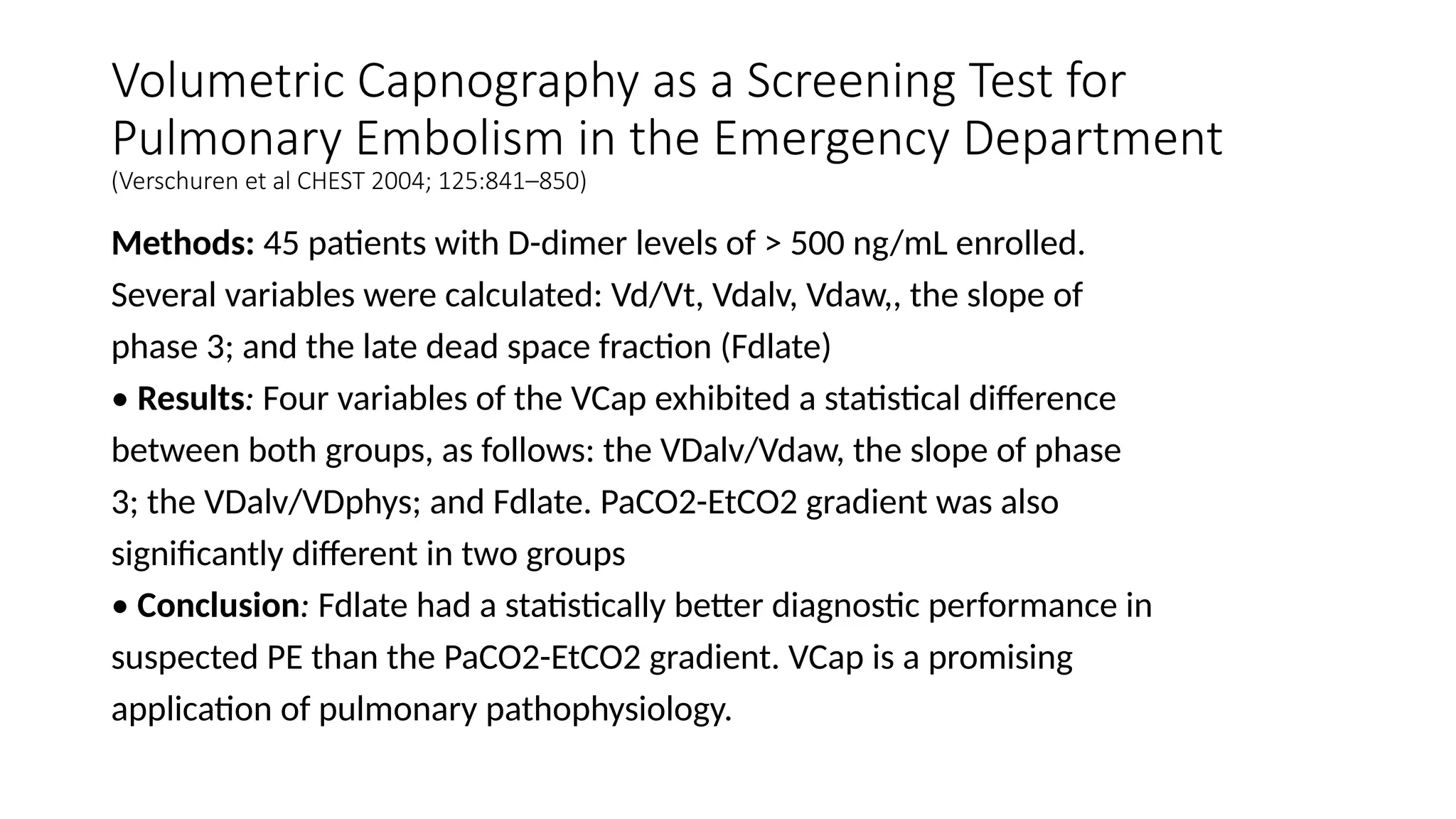
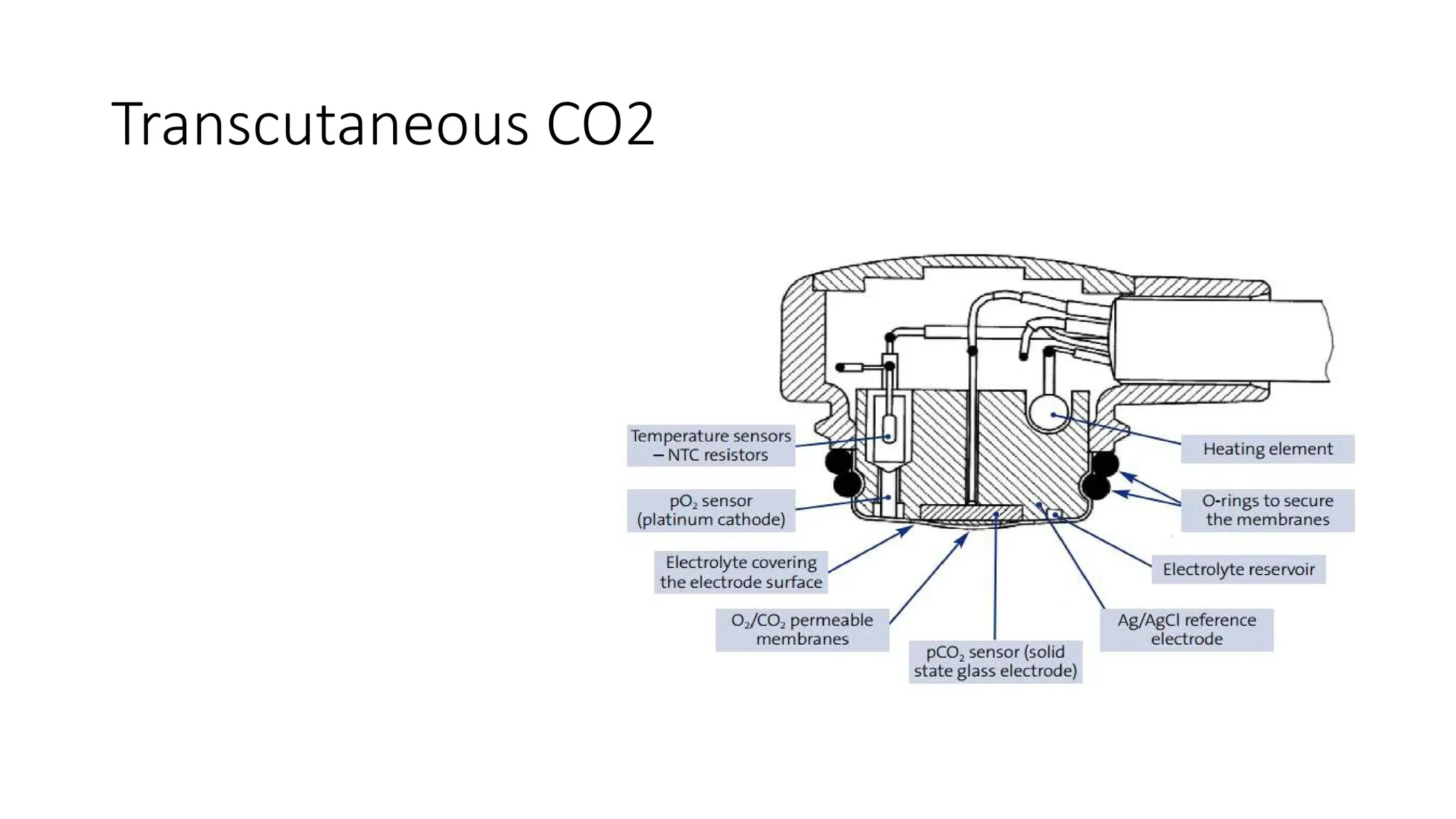
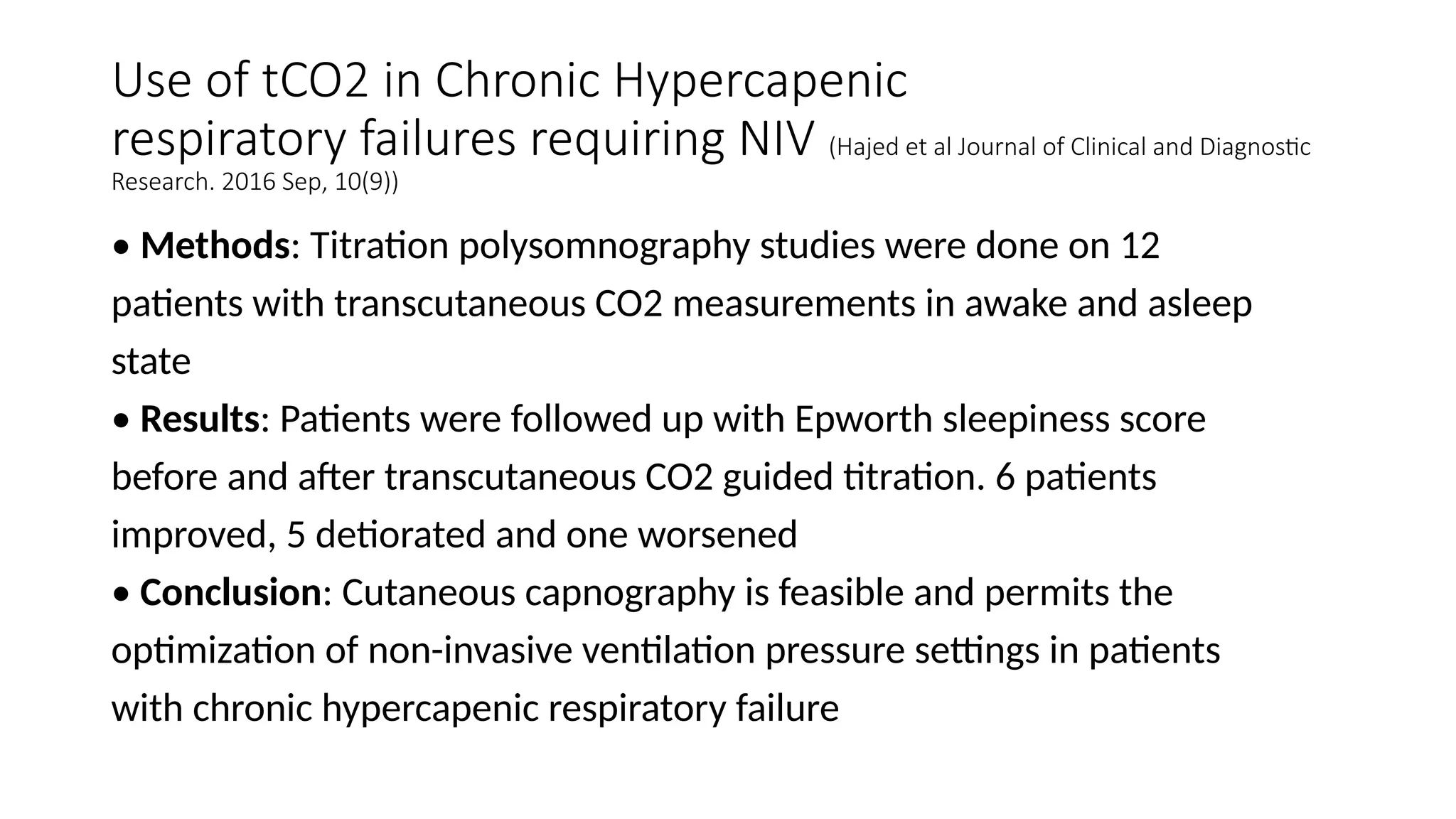
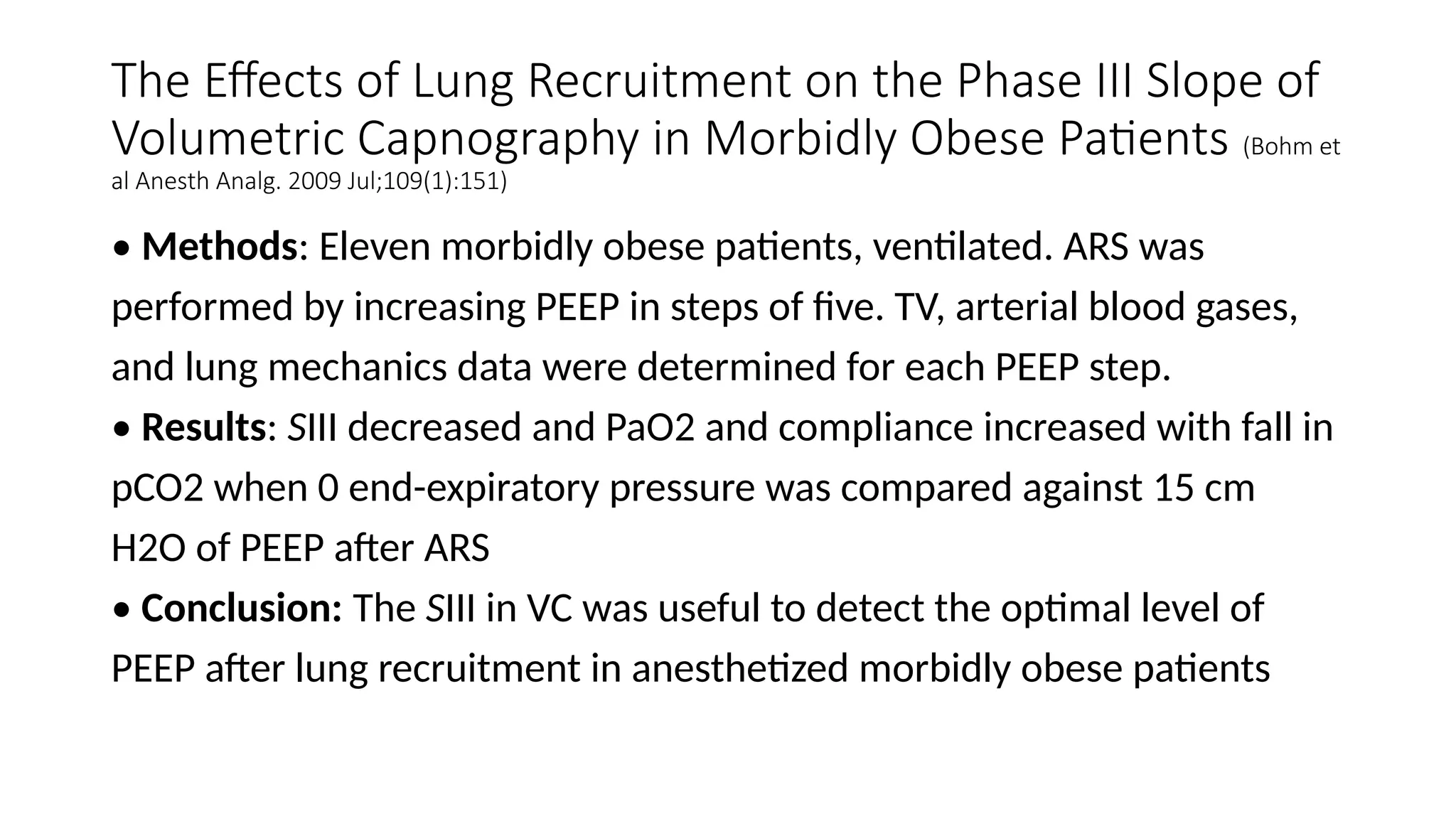
The document discusses capnometry and capnography, focusing on their importance in measuring CO2 levels during mechanical ventilation and assessing patient respiratory status. It details different monitoring techniques, abnormal capnographs, and guidelines for clinical application, emphasizing their roles in verifying airway placement, evaluating pulmonary circulation, and optimizing mechanical ventilation. Additionally, it covers contraindications, hazards, and the relevance of CO2 measurements in predicting patient outcomes during resuscitation and weaning processes.