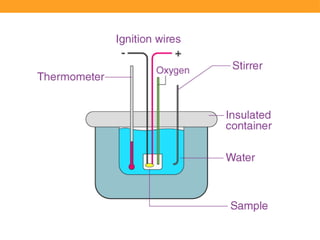
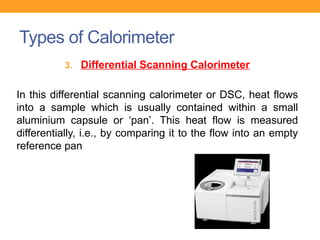
Calorimetry is the scientific study of measuring heat changes in physical and chemical processes, utilizing devices called calorimeters. Various types of calorimeters, such as bomb and direct calorimeters, are used in fields like nutrition, material science, and medicine to determine energy content, metabolism, and heat capacity. These instruments play a crucial role in applications, including nutritional labeling, metabolic studies, and industrial quality control.