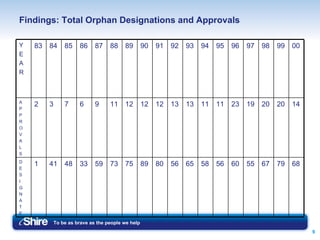
The document summarizes the Orphan Drug Act of 1983 and its impact. It provides incentives like 7 years of marketing exclusivity and tax credits to stimulate development of drugs for rare diseases defined as affecting fewer than 200,000 people. Since 1983, over 1000 designations and 200 product approvals have occurred. While the Act has met its objectives, concerns around the high costs of orphan drugs and determining appropriate access and reimbursement are discussed.