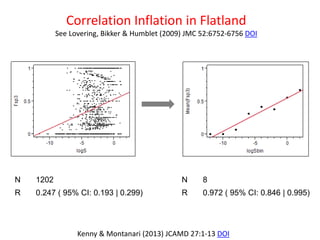
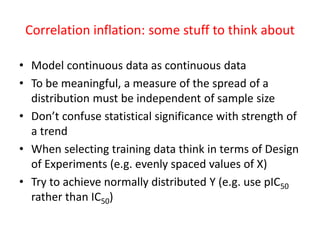
This document discusses issues with commonly used methods in molecular design and data analysis, including ligand efficiency metrics (LEMs). It argues that LEMs make unfounded assumptions about relationships between activity and risk factors. Instead, residuals from modeling activity data directly should be used to quantify how much activity exceeds trends, as they are invariant to choices like standard concentration and treat all risk factors consistently. The document advocates understanding data properties before analysis and avoiding practices like arbitrarily binning data that can distort correlations.



![TEP= [퐷푟푢푔푿,푡]푓푟푒푒 퐾푑
Target engagement potential (TEP)
A basis for pharmaceutical molecular design?
Design objectives
•Low Kdfor target(s)
•High (hopefully undetectable) Kdfor antitargets
•Ability to control[Drug(X,t)]free
Kenny, Leitão& MontanariJCAMD 2014 28:699-710 DOI](https://image.slidesharecdn.com/pwkbrazmedchem2014-141111135347-conversion-gate02/85/BrazMedChem2014-4-320.jpg)