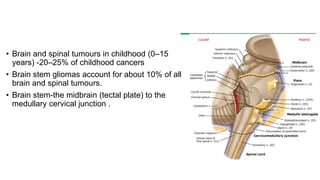
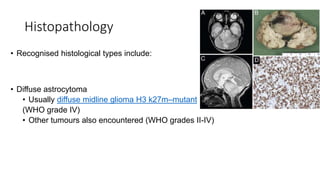
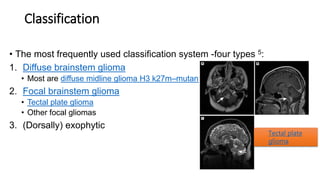
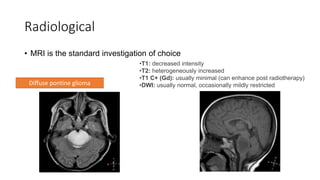
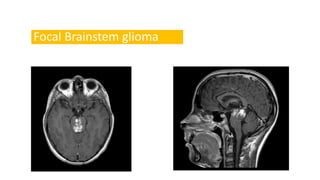
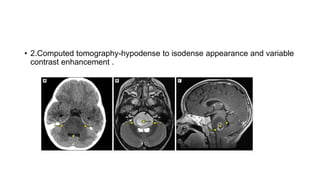
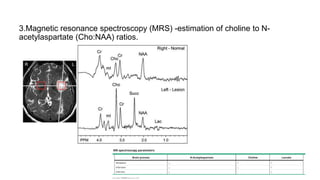
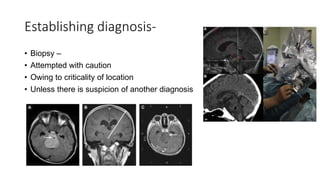
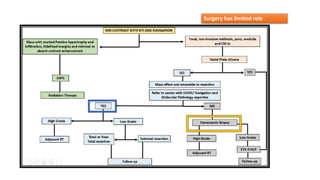
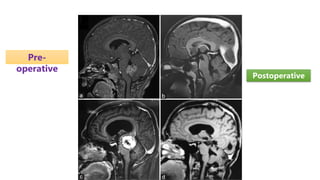
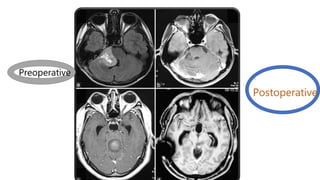
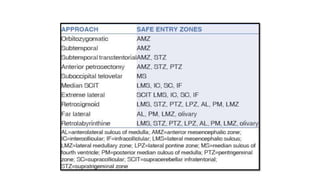
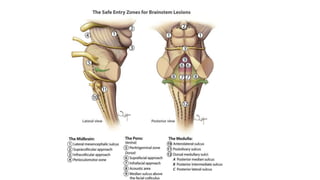
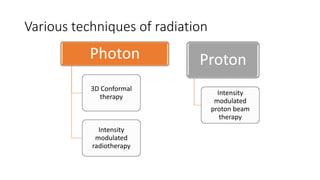
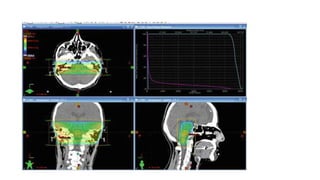
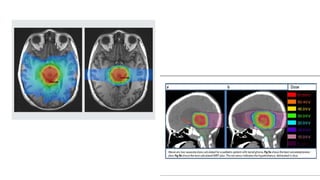
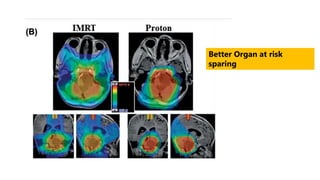
Brainstem gliomas account for about 10% of childhood brain and spinal tumors. They are challenging to treat due to their critical location. Radiation therapy plays a key role in management as surgery has limited effectiveness. Diffuse pontine gliomas, the most common type, have a terrible prognosis with over 90% of children dying within 2 years despite treatment. New immunotherapies and targeted therapies are being investigated as potential treatments to improve outcomes for these difficult to treat tumors.