Atomic physics
•Download as PPTX, PDF•
2 likes•1,992 views
atom model
Report
Share
Report
Share
Recommended
More Related Content
What's hot
What's hot (20)
Limitations OF Classical Physics and Birth Of Quantum Mechanics

Limitations OF Classical Physics and Birth Of Quantum Mechanics
Basic and fundamental of quantum mechanics (Theory)

Basic and fundamental of quantum mechanics (Theory)
Wave particle duality of light- A changing Notion in Science

Wave particle duality of light- A changing Notion in Science
Similar to Atomic physics
Similar to Atomic physics (20)
enc=encoded=PWW_dKfjbHrN9xq3SPtoL41DH0Bw5FrP4bCUo7yCo9hDDPhsJJZA_EXSSes=.pptx

enc=encoded=PWW_dKfjbHrN9xq3SPtoL41DH0Bw5FrP4bCUo7yCo9hDDPhsJJZA_EXSSes=.pptx
Compare and contrast the ideas that led to the Bohr model of the ato.pdf

Compare and contrast the ideas that led to the Bohr model of the ato.pdf
202006151236284892NK-Bohr Model of Hydrogen Atom.pdf

202006151236284892NK-Bohr Model of Hydrogen Atom.pdf
Bohrsmodelforhydrogenatom 141216074739-conversion-gate02

Bohrsmodelforhydrogenatom 141216074739-conversion-gate02
bohrsmodelforhydrogenatom-141216074739-conversion-gate02.pdf

bohrsmodelforhydrogenatom-141216074739-conversion-gate02.pdf
Recently uploaded
https://app.box.com/s/7hlvjxjalkrik7fb082xx3jk7xd7liz3TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...Nguyen Thanh Tu Collection
Mehran University Newsletter is a Quarterly Publication from Public Relations OfficeMehran University Newsletter Vol-X, Issue-I, 2024

Mehran University Newsletter Vol-X, Issue-I, 2024Mehran University of Engineering & Technology, Jamshoro
Recently uploaded (20)
ICT role in 21st century education and it's challenges.

ICT role in 21st century education and it's challenges.
TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
Sensory_Experience_and_Emotional_Resonance_in_Gabriel_Okaras_The_Piano_and_Th...

Sensory_Experience_and_Emotional_Resonance_in_Gabriel_Okaras_The_Piano_and_Th...
Unit 3 Emotional Intelligence and Spiritual Intelligence.pdf

Unit 3 Emotional Intelligence and Spiritual Intelligence.pdf
Food safety_Challenges food safety laboratories_.pdf

Food safety_Challenges food safety laboratories_.pdf
Fostering Friendships - Enhancing Social Bonds in the Classroom

Fostering Friendships - Enhancing Social Bonds in the Classroom
Exploring_the_Narrative_Style_of_Amitav_Ghoshs_Gun_Island.pptx

Exploring_the_Narrative_Style_of_Amitav_Ghoshs_Gun_Island.pptx
Plant propagation: Sexual and Asexual propapagation.pptx

Plant propagation: Sexual and Asexual propapagation.pptx
NO1 Top Black Magic Specialist In Lahore Black magic In Pakistan Kala Ilam Ex...

NO1 Top Black Magic Specialist In Lahore Black magic In Pakistan Kala Ilam Ex...
Atomic physics
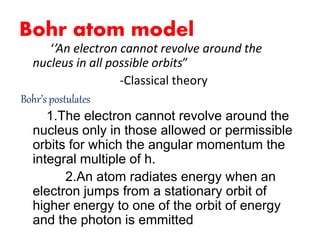
- 1. Bohr atom model ‘’An electron cannot revolve around the nucleus in all possible orbits” -Classical theory Bohr’s postulates 1.The electron cannot revolve around the nucleus only in those allowed or permissible orbits for which the angular momentum the integral multiple of h. 2.An atom radiates energy when an electron jumps from a stationary orbit of higher energy to one of the orbit of energy and the photon is emmitted
- 2. By using Bohr formulae we can calculate a)radii of the stationary orbits b)total energy of the electron in the orbit SPECTRAL SERIES OF THE HYDROGEN ATOM: a) Lyman series b) Balmer series c) paschen series d) Brackett series e) Pfund series
- 3. CRITICAL POTENTIALS: The least energy expressed in electron volts required to excite a free neutral atom from its ground state to a higher state is called critical potential EXCITATION POTENTIALS: The energy in electron volts is required to raise an atom from its normal state into an excited state is called potential of the state IONISATION POTENTIALS: The energy required to remove an electron from a given orbit to an infinite distance from the nucleus
- 4. Experimental determination of critical potentials Frank and Hertz’s method This experiment shows that the energy lost by the electron in its collision with the mercury atom reappears as a quantum of energy and its wavelength Davis and Goucher’s method This experiment is used to subject to a systematic error on account of the initial velocity of emission of the electrons from the hot filament.
- 5. SOMMERFELD RELATIVISTIC ATOM MODEL Sommerfeld introduced two main modifications in the Bohr’s theory: 1) The path of the electron around the nucleus , in general, is an ellipse with the nucleusat one of the foci.The circular orbits of Bohr are a special caseof this . 2)The velocity of the electron moving in an ellipticalorbit varies considerably at different parts of the orbit.This causes relativistic variation in the mass of the moving electron.Therefore he took into account the relativistic variation of the massof the electron with velocity .Hence this is known as THERELATIVISTIC ATOM-MODEL.
- 6. Vector atom model 1) Bohr’s theory was able to explain the series of the simplest hydrogen atom. 2) The older theories were inadequate to explain new discoveries like the zeeman effect and stark effect in which the spectral lines slit under the influence of magnetic and electric fields 3) Another drawback of the Bohr model was that it could not explain how the electrons in an atom were distributed around the nucleus
- 7. tHETWO DISTINCT FEATURES OF THE VECTOR ATOM MODEL ARE: 1) The conception of the spatial quantisation 2) The spinning electron hypothesis
- 8. There are 7 types of quantum numbers associated with the vector atom model • Principal quantum number • Orbital quantum number • Spin quantum number • Total angular quantum number • Magnetic orbital quantum number • Magnetic spin quantum number • Magnetic total angular momentum quantum number
- 9. Coupling schemes 1) L-S coupling In this type all the orbital angular momentum of the vectors combine to form a resultant L and independently, all their spin angular momentum vectors combine to form a resultant S. 1) j-j coupling It is employed with the interactions between spin and orbital vectors of the each electron
- 10. Pauli’s exclusion principle No two electrons in an atom exist in thr same quantum state. “No two electrons in an isolated atom may have the same four quantum numbers”
- 11. Periodic classification of elements The periodic table is an arrangementof differentelements thatexist the nature,based on theirchemical properties and theatomic number Thereare 7 periods and 8 groups in a periodic table
- 12. Magnetic dipole moment They are of 2 types Namely • due to orbital motion of the elecctron • due to spin
- 13. Stern and Gerlach experiment This experiment is based on the behaviour f a magnetic dipole in a non- uniform magnetic field, the dipole experiences the torque that tends to align the dipole parellel to the field