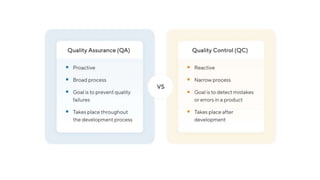
Quality assurance aims to maintain a high level of quality in products and services. It involves audits and assessments to detect and correct any issues that do not meet established standards. Quality assurance looks at all stages of development and production to ensure each aspect will deliver a quality customer experience, while quality control focuses more narrowly on checking the finished product. Good manufacturing practices also help ensure products are consistently manufactured to quality standards through documented processes, validated equipment and facilities, trained personnel, and other measures.