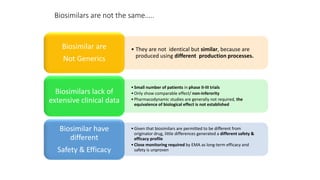
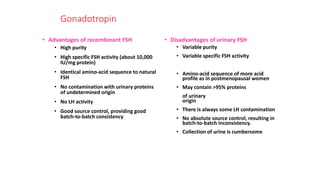
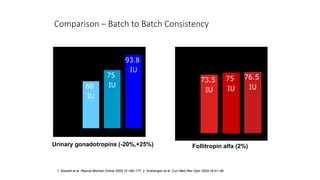
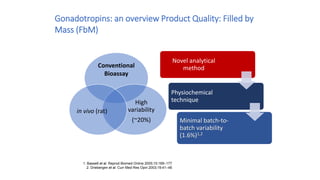
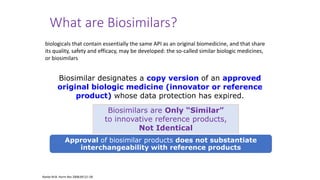
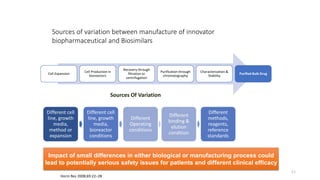
The document discusses biosimilars, specifically recombinant follicle-stimulating hormone (rFSH), highlighting their advantages over urinary FSH in terms of purity, efficacy, and batch consistency. It notes that while biosimilars are designed to be similar to original biologics, they are not identical and can have different safety and efficacy profiles due to variations in production processes. The importance of monitoring and further proving biosimilars' safety and effectiveness is emphasized, especially in assisted reproductive technology treatments.






![Concerns
Loss of efficacy
Immune effects such as anaphylaxis or allergic reactions
Can be severe and potentially lethal
Nephrol Dial Transplant (2006) 21 [Suppl 5]: v9–v12; NATURE BIOTECHNOLOGY 2004;22(11):1357-9
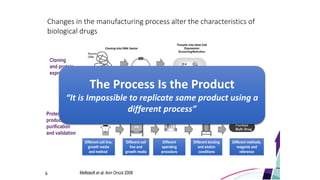
Even if the biosimilar product has the same gene
sequence, vector, host cell line, culture conditions
and purification methods as the innovative protein,
it can still differ substantially in its biological and
clinical properties.](https://image.slidesharecdn.com/biosimilar-230314053501-c2fb1e49/85/Biosimilar-pptx-14-320.jpg)