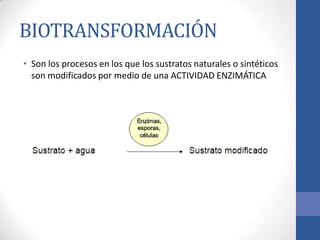
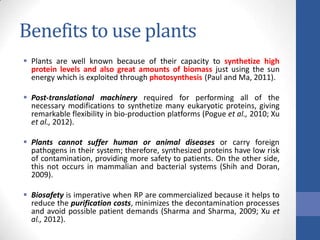
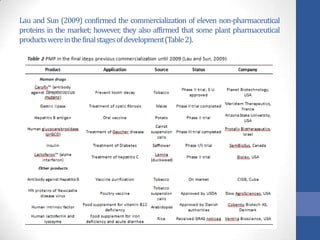
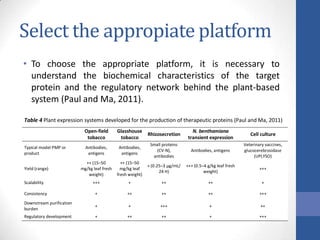
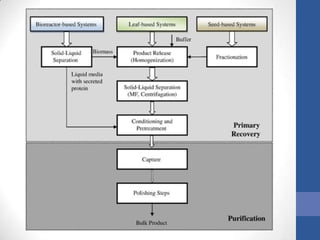
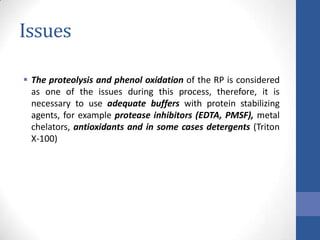
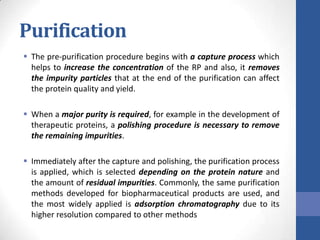
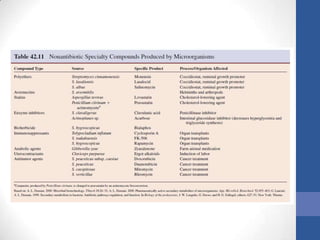
This document discusses bioprocesses and describes the process of beer production. It begins with definitions of bioprocesses and bioprocess engineering. Beer production occurs through alcoholic fermentation, a fermentation process without oxygen used by microorganisms like yeast to convert sugars like glucose into ethanol and carbon dioxide. The yeast species used for beer production is Saccharomyces cerevisiae. Fermentation has been used since ancient times to produce products like beer and wine. Current bioprocesses have various industrial uses such as producing cosmetics, cleaning products, biofuels, biological pesticides, recombinant vaccines, industrial enzymes, pharmaceuticals like antibiotics, and more.