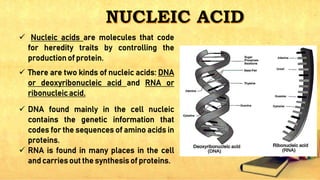
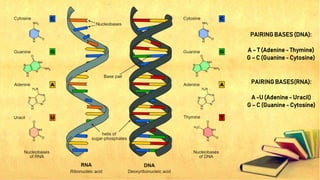
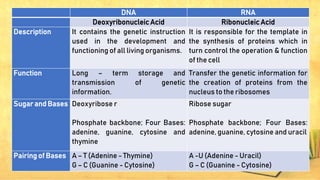
Carbohydrates, lipids, proteins, and nucleic acids are the four major macromolecules that make up all living things. Carbohydrates include monosaccharides like glucose, fructose, and galactose; disaccharides formed from two monosaccharides such as sucrose and lactose; and polysaccharides like starch, cellulose, and glycogen. Lipids contain fats, oils, and cholesterol and are used to store energy. Proteins are made of amino acids and perform important structural and functional roles in the body. Nucleic acids DNA and RNA contain genetic information and help direct protein synthesis.