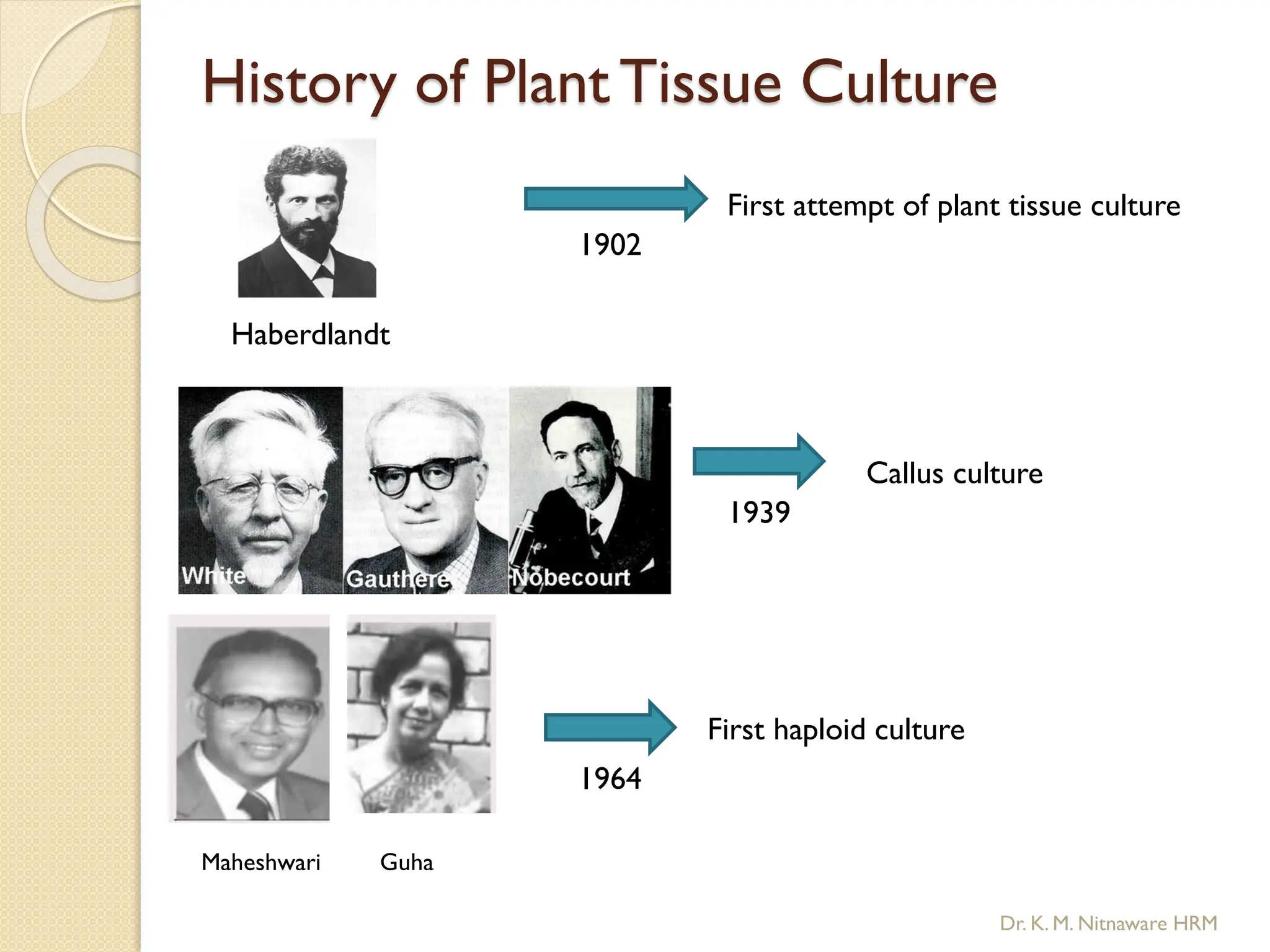
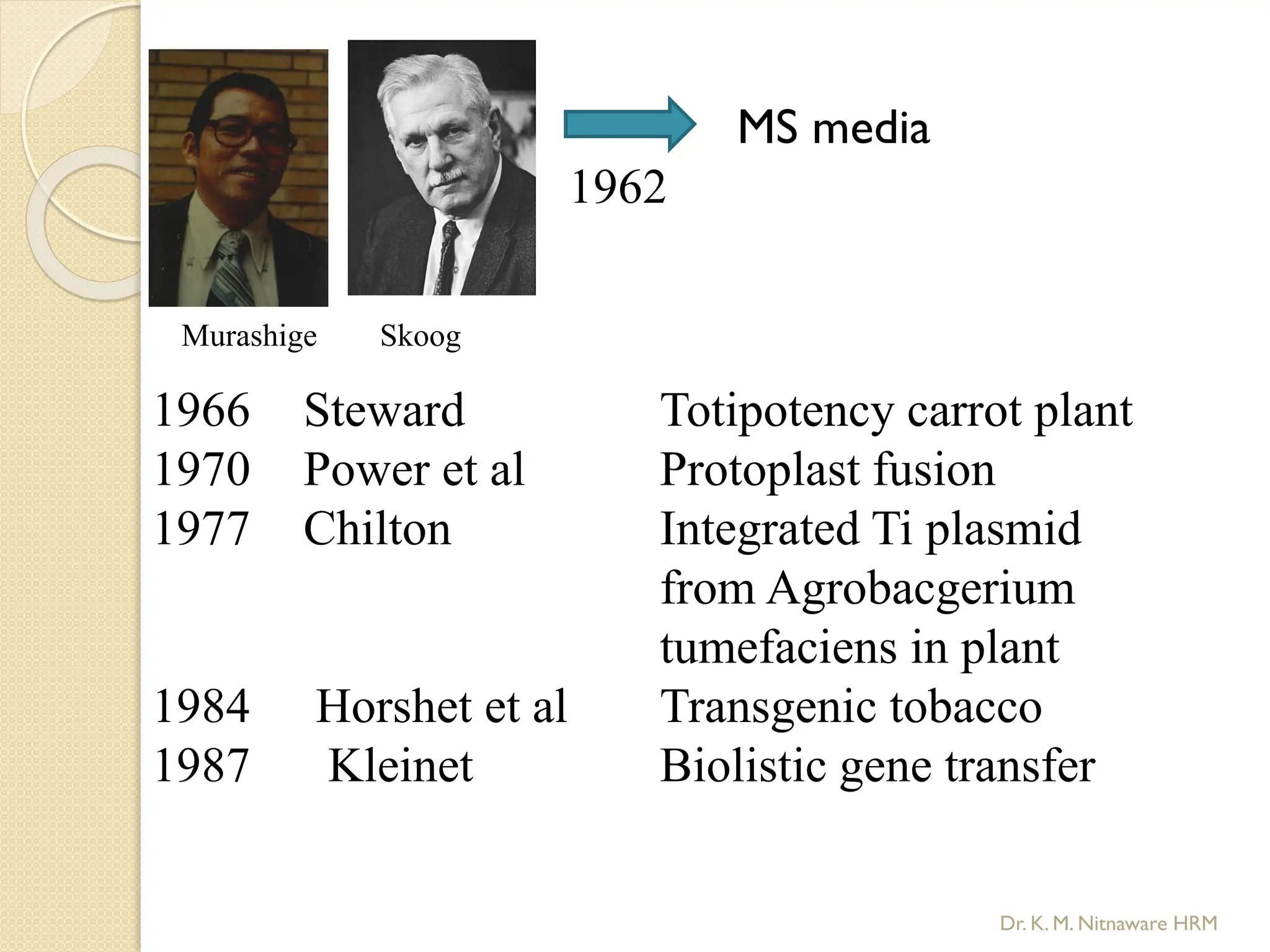
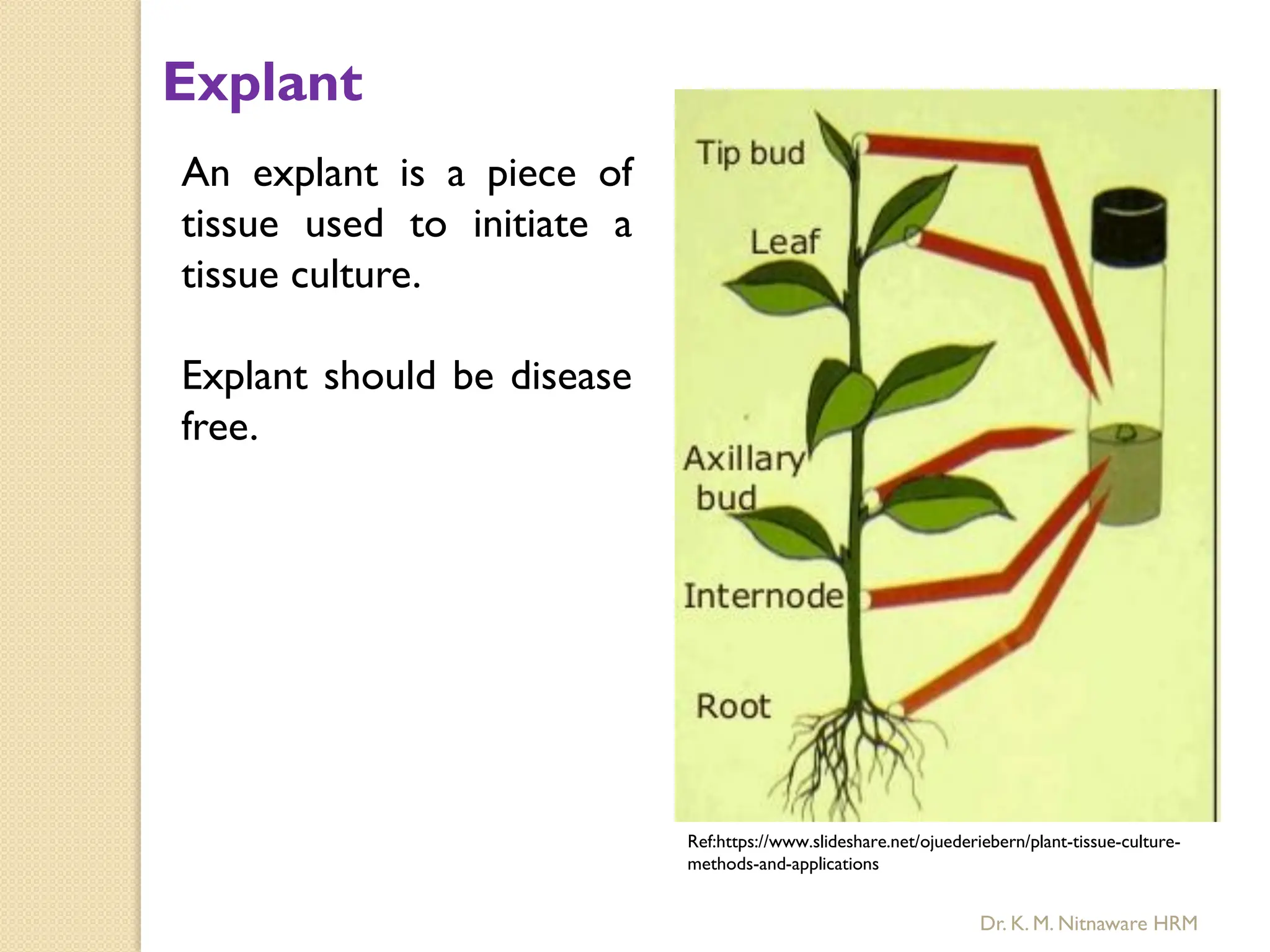
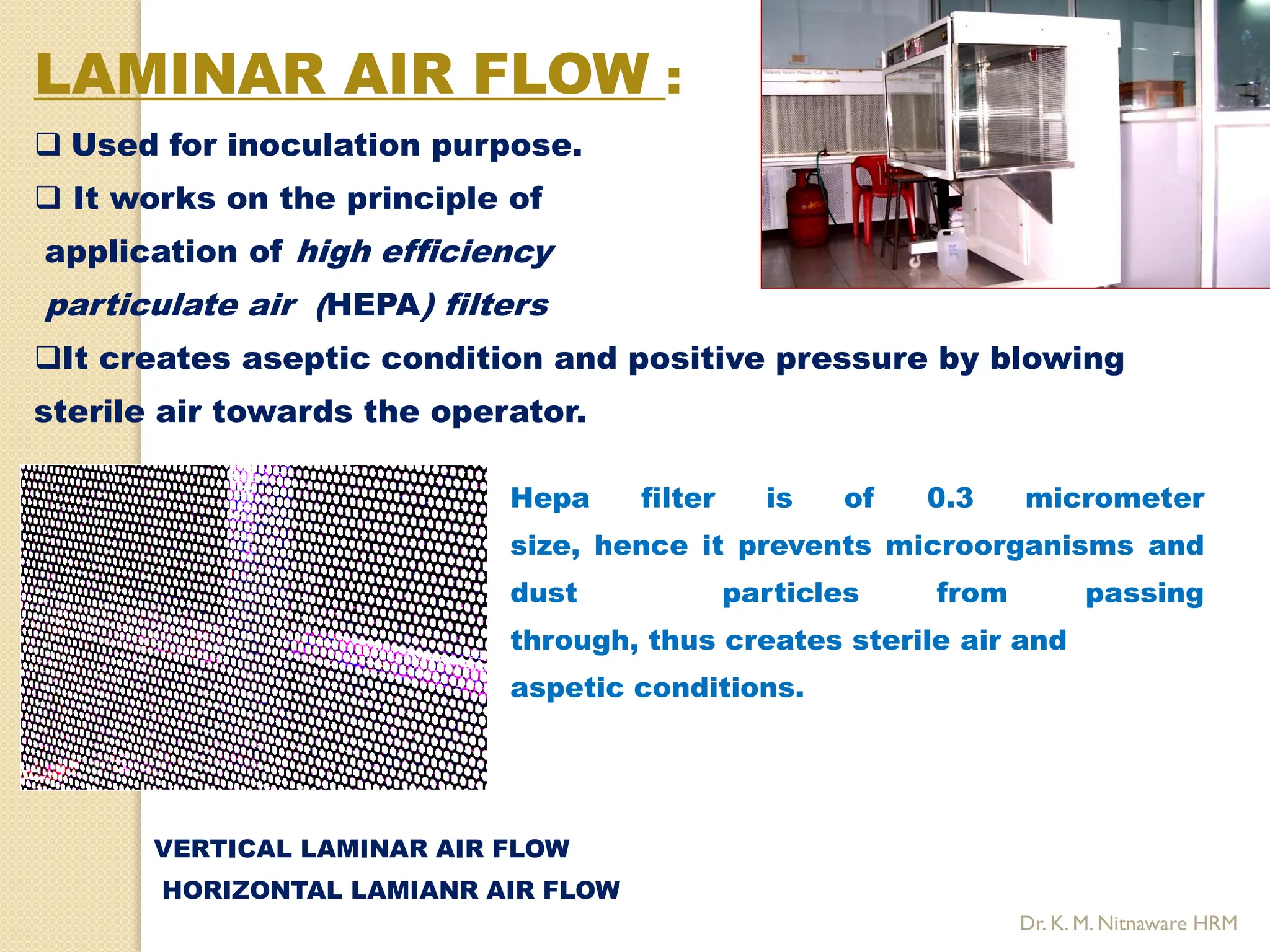
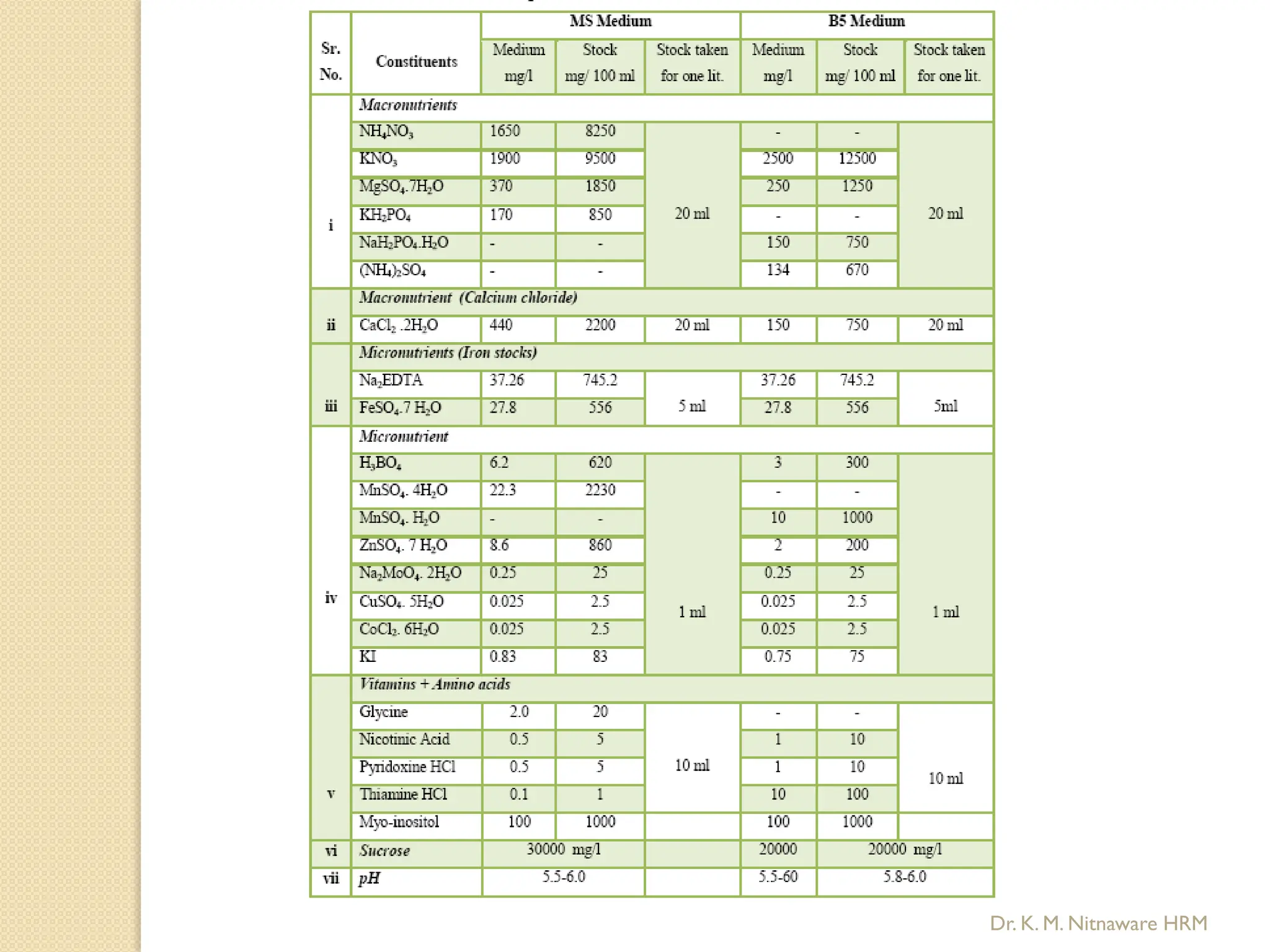
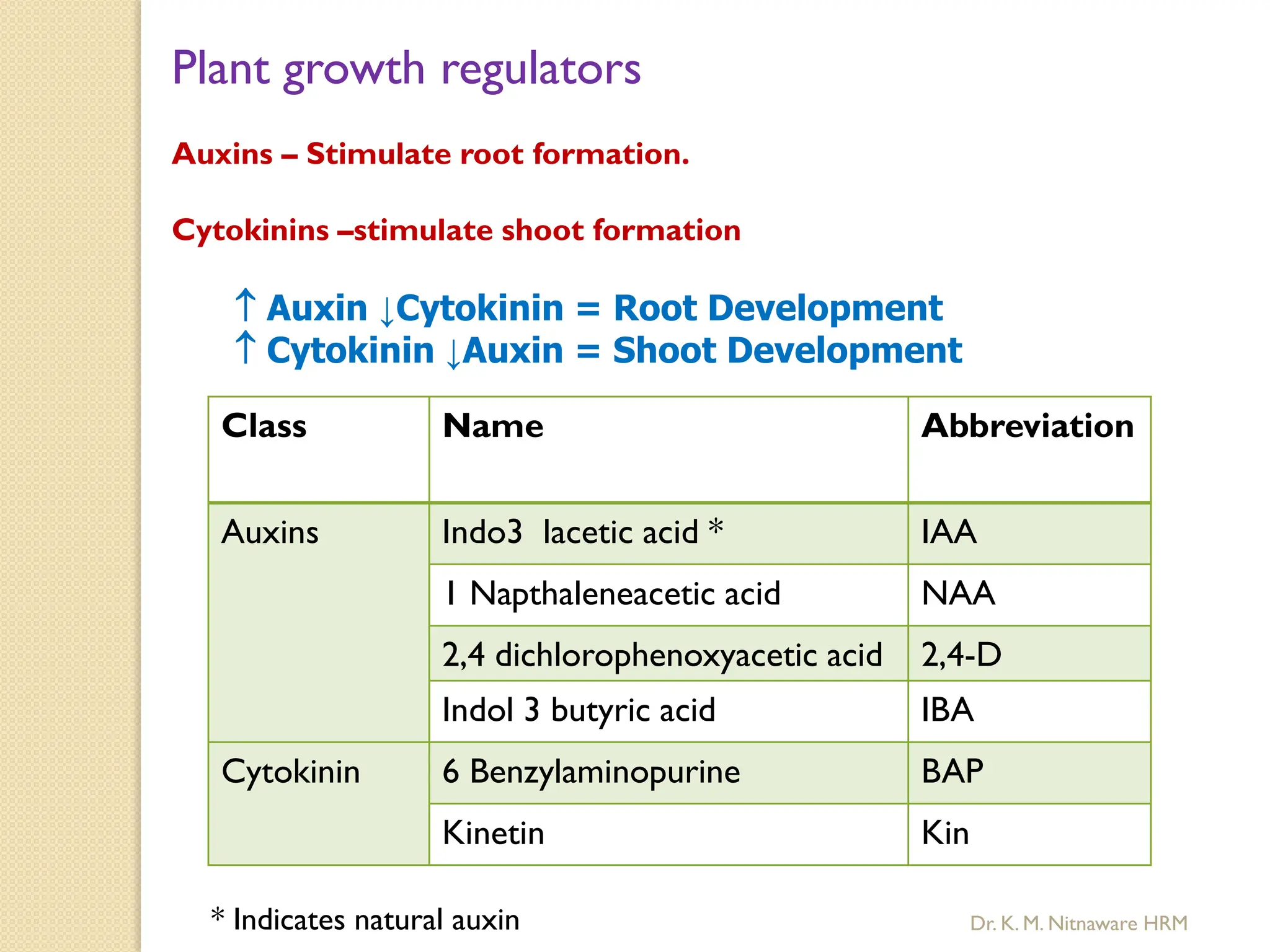
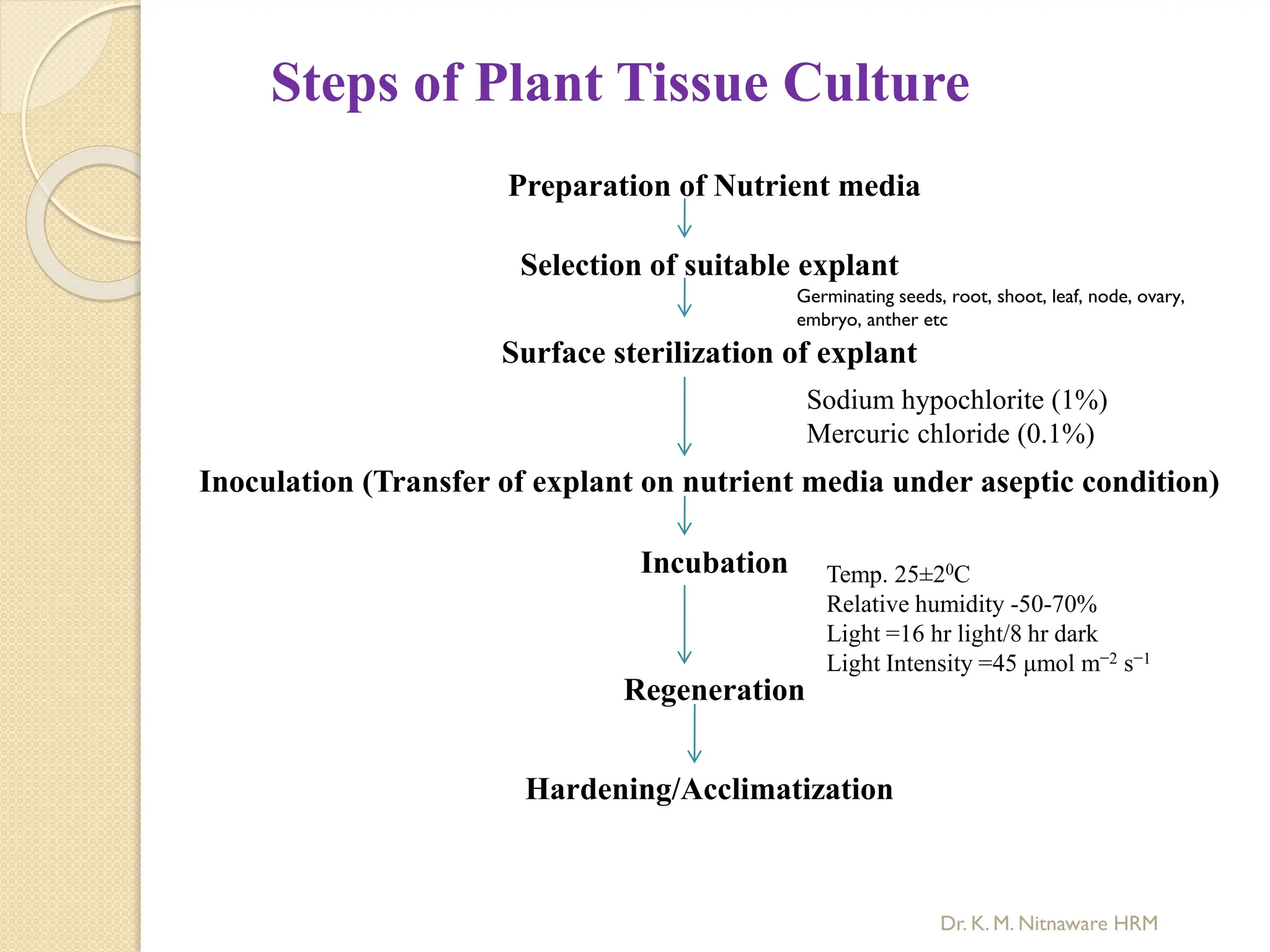
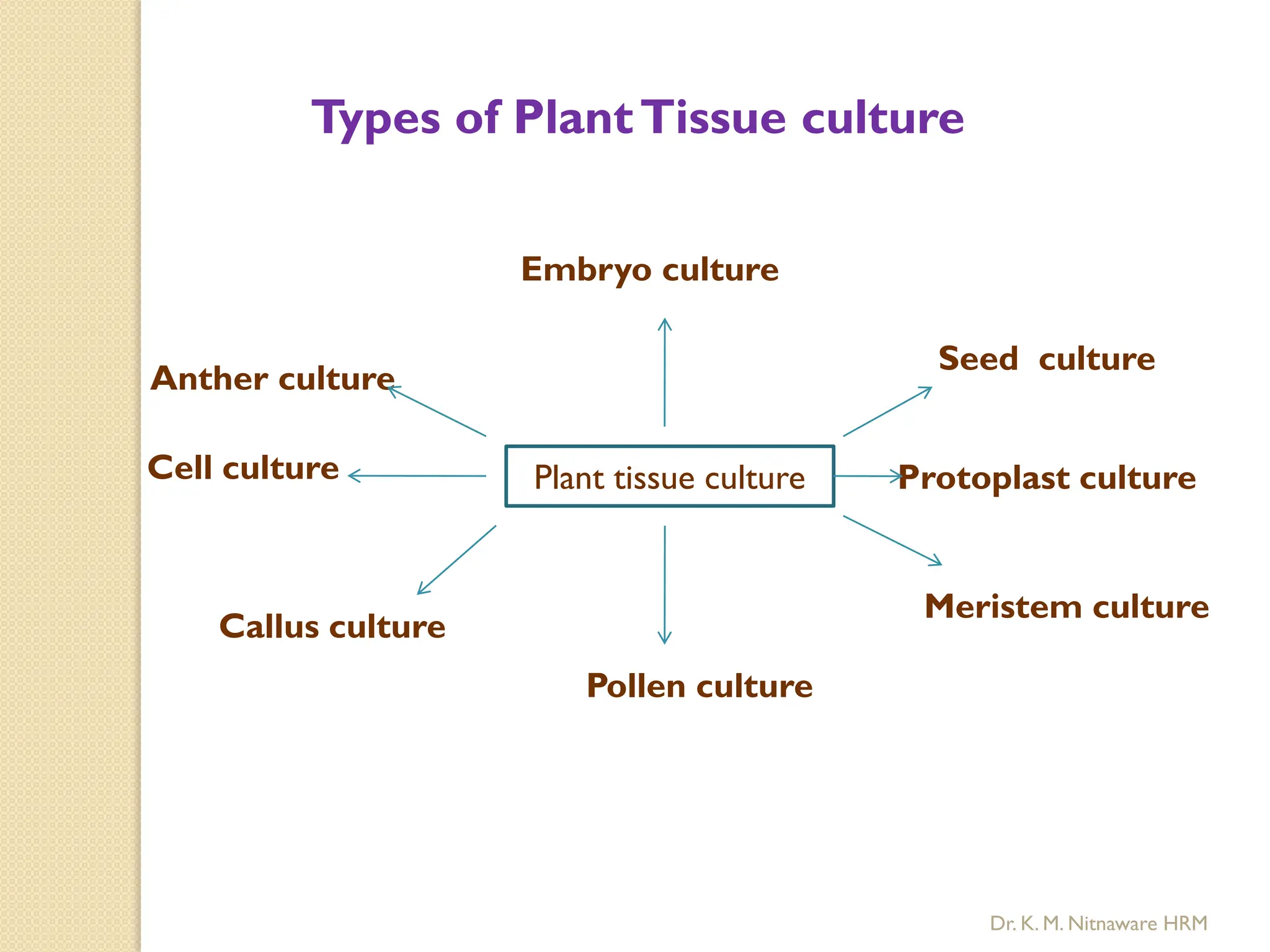
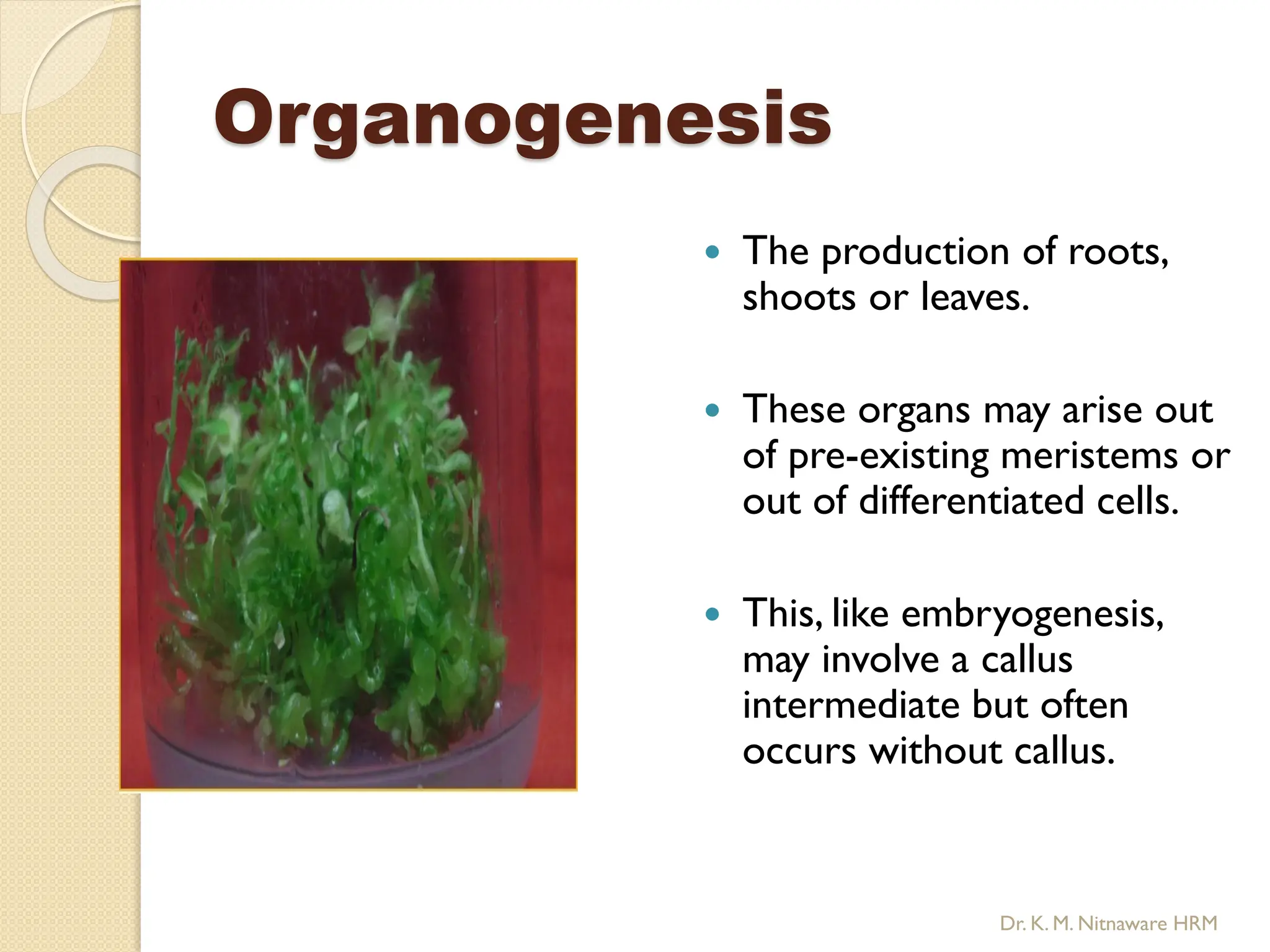
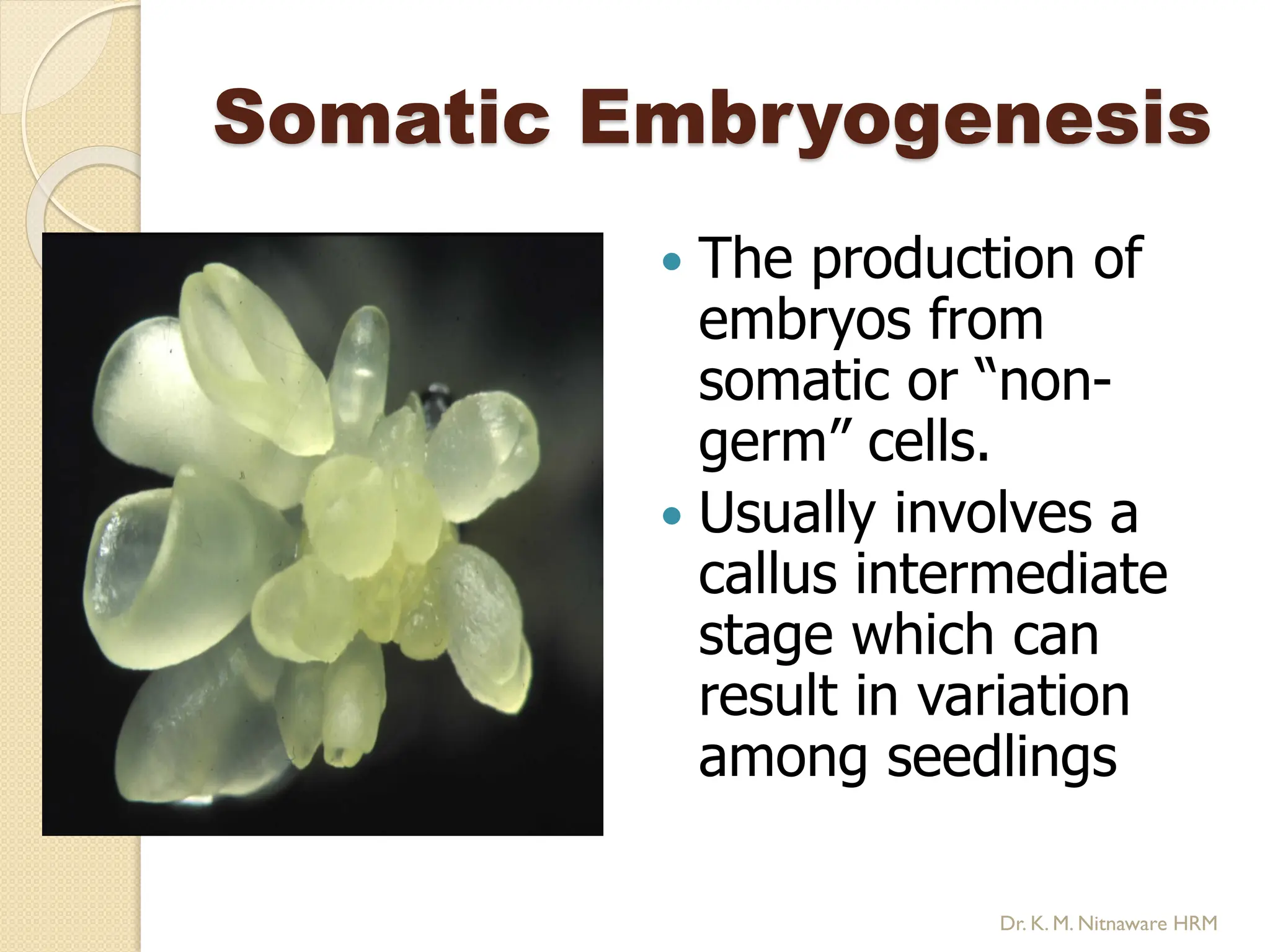
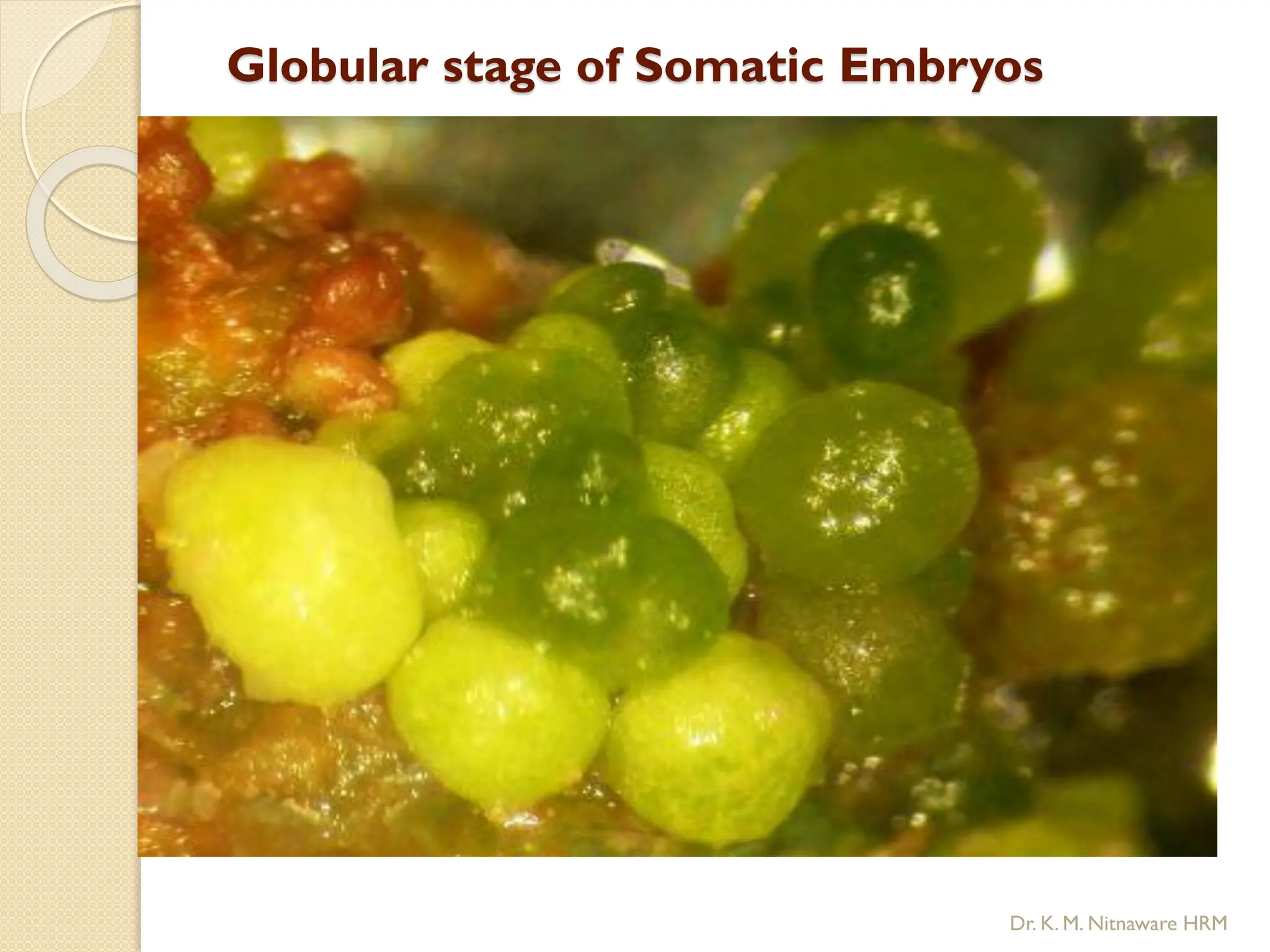
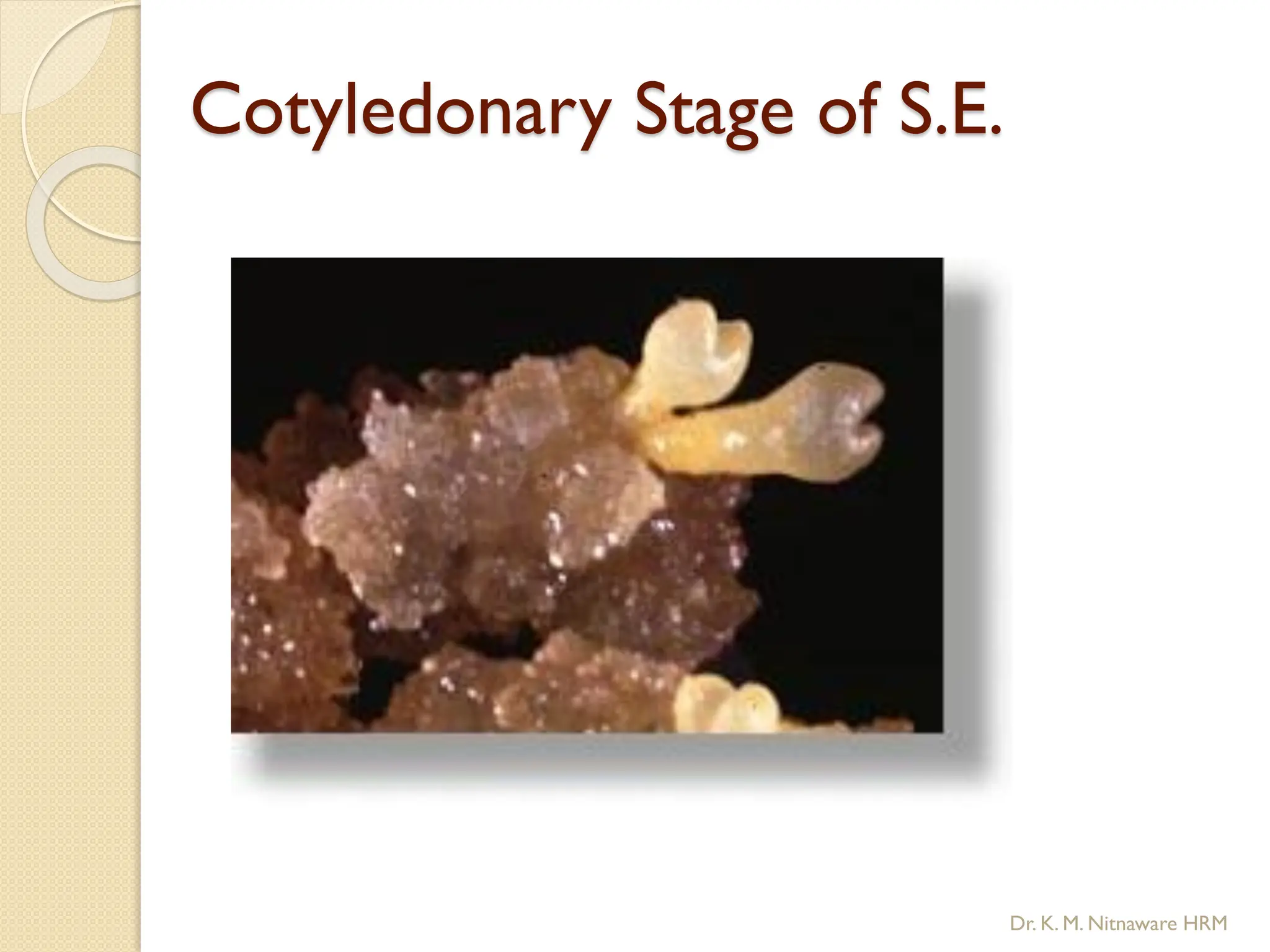
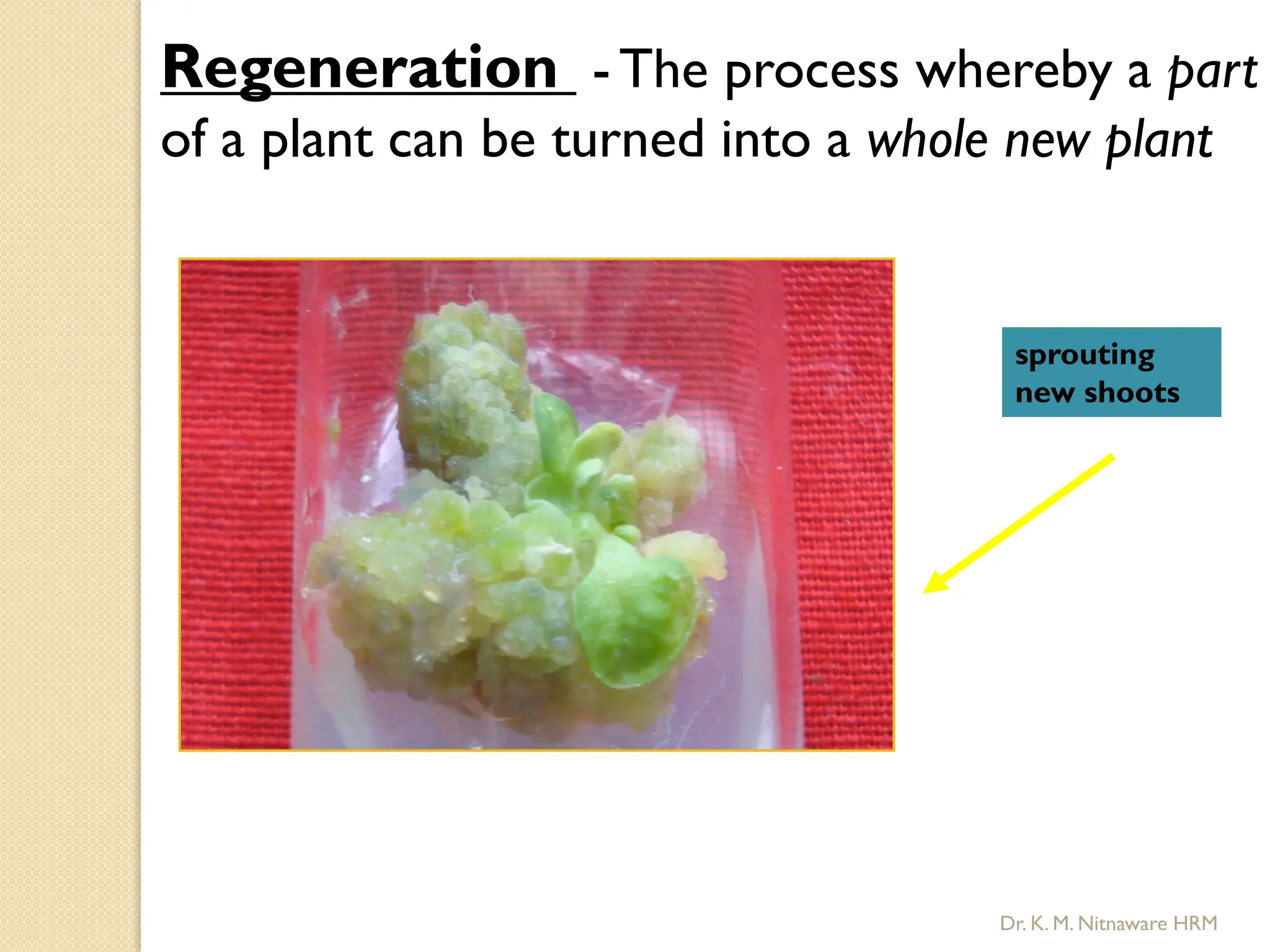
The document provides an overview of plant tissue culture, detailing its definition, history, and essential terminology such as explant, callus, and micropropagation. It outlines the requirements for tissue culture, including aseptic conditions, appropriate equipment, and nutrient media, as well as the steps involved in the process and the types of cultures like organogenesis and somatic embryogenesis. Additionally, it highlights the importance of plant tissue culture in producing improved crop varieties and disease-free propagules.