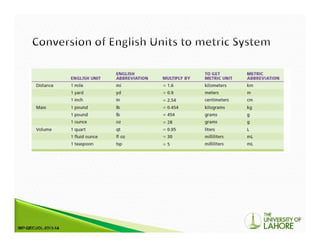
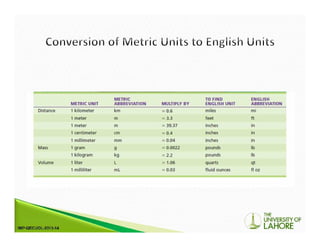
The document outlines the International System of Units (SI) and its significance in modern measurements alongside customary units in the US. It discusses the creation and use of laboratory solutions, dilutions, and the preparation of specific reagent water types like CLRW for analytical procedures. It also describes the process for making serial dilutions and titers, which are useful for immunological tests and determining the strength of specific components in serum samples.