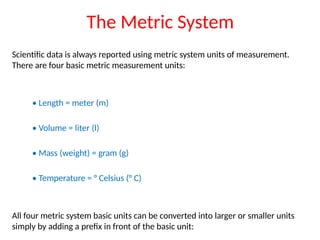
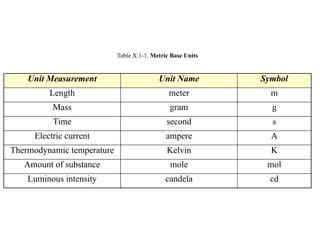
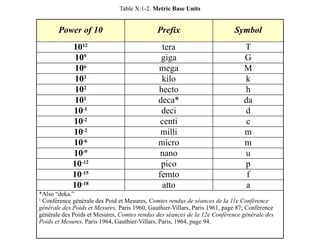
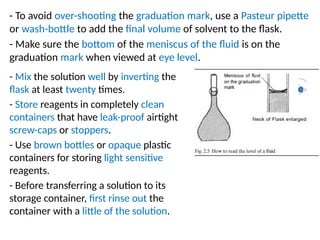
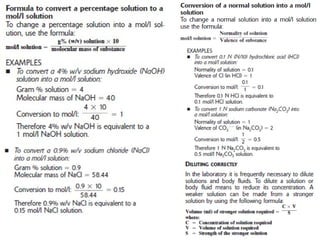
The document discusses the metric system and the preparation of reagents, highlighting the advantages and disadvantages of both the metric and English systems of measurement. It details the proper techniques for preparing solutions and reagents, emphasizing accuracy, labeling, and storage considerations, while also explaining common errors in preparation. Additionally, it provides guidance on calculating concentrations for molar solutions and the characteristics of hygroscopic and deliquescent chemicals.