Embed presentation
Downloaded 33 times

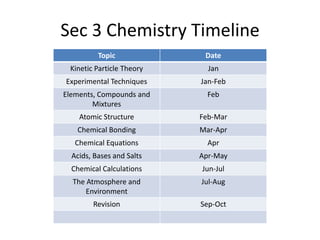
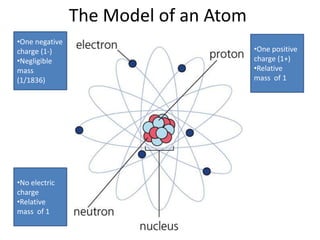
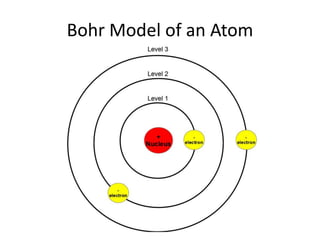
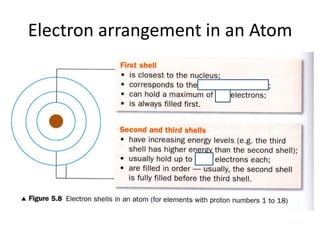

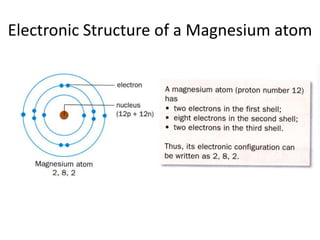
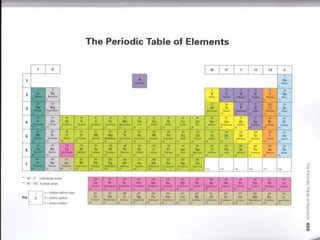
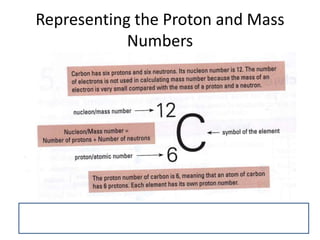
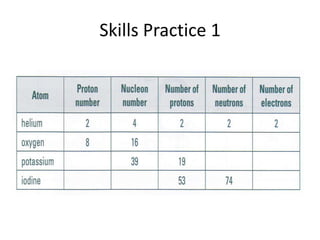
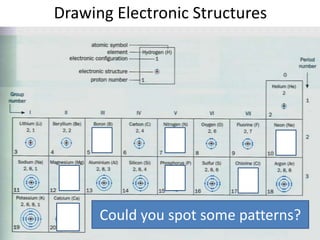
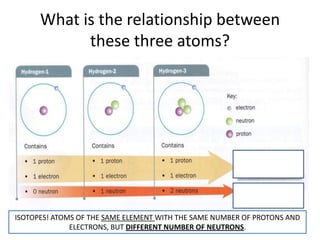
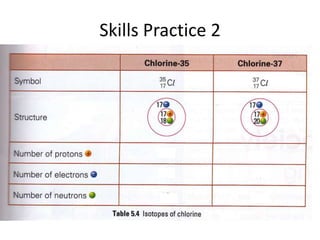
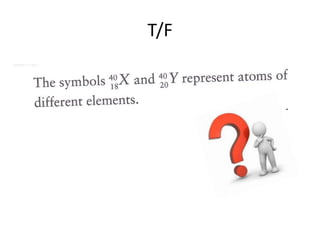
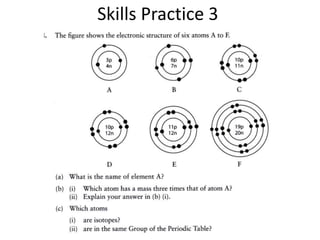

The document provides a timeline and overview of topics to be covered in a Sec 3 Chemistry class throughout the year, beginning with Kinetic Particle Theory in January and ending with Revision in September-October. Key topics included are Experimental Techniques, Atomic Structure, Chemical Bonding, Chemical Equations, Acids Bases and Salts, and Chemical Calculations. The document also provides details on Atomic Structure, including subatomic particles, Bohr's model of the atom, electron configuration and arrangement, and representing proton and mass numbers.


















