Complexometric-Titrations part 2.ppt
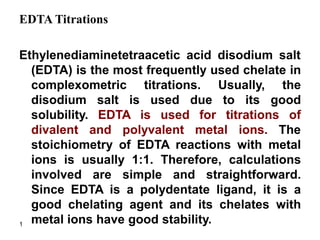
- 1. 1 EDTA Titrations Ethylenediaminetetraacetic acid disodium salt (EDTA) is the most frequently used chelate in complexometric titrations. Usually, the disodium salt is used due to its good solubility. EDTA is used for titrations of divalent and polyvalent metal ions. The stoichiometry of EDTA reactions with metal ions is usually 1:1. Therefore, calculations involved are simple and straightforward. Since EDTA is a polydentate ligand, it is a good chelating agent and its chelates with metal ions have good stability.
- 2. 2
- 3. 3 EDTA Equilibria EDTA can be regarded as H4Y where in solution we will have, in addition to H4Y, the following species: H3Y-, H2Y2-, HY3-, and Y4-. The amount of each species depends on the pH of the solution where: a4 = [Y4-]/CT where: CT = [H4Y] + [H3Y-] + [H2Y2-] + [HY3-] + [Y4-] The species Y4- is the ligand species in EDTA titrations and thus should be looked at carefully.
- 4. 4
- 5. 5 The Formation Constant Reaction of EDTA with a metal ion to form a chelate is a simple reaction. For example, EDTA reacts with Ca2+ ions to form a Ca-EDTA chelate forming the basis for estimation of water hardness. The reaction can be represented by the following equation: Ca2+ + Y4- = CaY2- kf = 5.0x1010 Kf = [CaY2-]/[Ca2+][Y4-] The formation constant is very high and the reaction between Ca2+ and Y4- can be considered quantitative. Therefore, if equivalent amounts of Ca2+ and Y4- were mixed together, an equivalent amount of CaY2- will be formed.
- 6. 6 Formation Constants for EDTA Complexes Cation KMY Cation KMY Ag+ 2.1 x 107 Cu2+ 6.3 x 1018 Mg2+ 4.9 x 108 Zn2+ 3.2 x 1016 Ca2+ 5.0 x1010 Cd2+ 2.9 x 1016 Sr2+ 4.3 x 108 Hg2+ 6.3 x 1021 Ba2+ 5.8 x 107 Pb2+ 1.1 x 1018 Mn2+ 6.2 x1013 Al3+ 1.3 x 1016 Fe2+ 2.1 x1014 Fe3+ 1.3 x 1025 Co2+ 2.0 x1016 V3+ 7.9 x 1025 Ni2+ 4.2 x1018 Th4+ 1.6 x 1023
- 7. 7 Minimum pH for effective titrations of various metal ions with EDTA.
- 8. 8
- 9. 9 Titration Curves In most cases, a titration is performed by addition of the titrant (EDTA) to the metal ion solution adjusted to appropriate pH and in presence of a suitable indicator. The break in the titration curve is dependent on: 1. The value of the formation constant. 2. The concentrations of EDTA and metal ion. 3. The pH of the solution As for acid-base titrations, the break in the titration curve increases as kf increases and as the concentration of reactants is increased. The pH effect on the break of the titration curve is such that sharper breaks are obtained at higher pH values.
- 10. 10
- 11. 11 Indicators The indicator is usually a weaker chelate forming ligand. The indicator has a color when free in solution and has a clearly different color in the chelate. The following equilibrium describes the function of an indicator (H3In) in a Mg2+ reaction with EDTA: MgIn- (Color 1) + Y4- D MgY2- + In3- (Color 2)
- 12. 12
- 13. 13
- 14. 14 Example Calculate the pCa of a solution at pH 10 after addition of 100 mL of 0.10 M Ca2+ to 100 mL of 0.10 M EDTA. a4 at pH 10 is 0.35. kf = 5.0x1010 Solution Ca2+ + Y4- = CaY2- mmol Ca2+ = 0.10 x 100 = 10 mmol EDTA = 0.10 x 100 = 10 mmol CaY2- = 10 [CaY2-] = 10/200 = 0.05 M Therefore, Ca2+ will be produced from partial dissociation of the complex
- 15. 15 Ca2+ + Y4- D CaY2- CT = [H4Y] + [H3Y-] + [H2Y2-] + [HY3-] + [Y4-] Kf = [CaY2-]/[Ca2+]a4CT [Ca2+] = CT 5.0x1010 = 0.05/([Ca2+]2 x 0.35) [Ca2+] = 1.7x10-6 M pCa = 5.77 Using the same type of calculation we are used to perform, one can write the following:
- 16. 16 Kf = [CaY2-]/[Ca2+][Y4-] 5.0x1010 = (0.05 – x)/(x* a4 x) assume that 0.05>>x x = 1.7x10-6 Relative error = (1.7x10-6/0.05) x 100 = 3.4x10-3% [Ca2+] = 1.7x10-6 M pCa = 5.77
- 17. 17 Calculate the titer of a 0.100 M EDTA solution in terms of mg CaCO3 (FW = 100.0) per mL EDTA The EDTA concentration is 0.100 mmol/mL, therefore, the point here is to calculate the mg CaCO3 reacting with 0.100 mmol EDTA. We know that EDTA reacts with metal ions in a 1:1 ratio. Therefore 0.100 mmol EDTA will react with 0.100 mmol CaCO3. mmol CaCO3 = mmol EDTA mg CaCO3/100 = 0.1 * 1 mg CaCO3 = 10.0
- 18. 18 Example An EDTA solution is standardized against high purity CaCO3 by dissolving 0.3982 g of CaCO3 in HCl and adjusting the pH to 10. The solution is then titrated with EDTA requiring 38.26 mL. Find the molarity of EDTA. Solution EDTA reacts with metal ions in a 1:1 ratio. Therefore, mmol CaCO3 = mmol EDTA mg/FW = Molarity x VmL 398.2/100.0 = M x 38.26, MEDTA = 0.1041
- 19. 19 Example Find pCa in a 100 mL solution of 0.10 M Ca2+ at pH 10 after addition of 0, 25, 50, 100, 150, and 200 mL of 0.10 M EDTA. a4 at pH 10 is 0.35. kf = 5x1010 Solution Again, we should remember that EDTA reactions with metal ions are 1:1 reactions. Therefore, we have: Ca2+ + Y4- D CaY2- kf = 5.0x1010 1. After addition of 0 mL EDTA [Ca2+] = 0.10 M pCa = 1.00
- 20. 20 2. After addition of 25 mL EDTA Initial mmol Ca2+ = 0.10 x 100 = 10 mmol EDTA added = 0.10 x 25 = 2.5 mmol Ca2+ left = 10 – 2.5 = 7.5 [Ca2+]left = 7.5/125 = 0.06 M In fact, this calcium concentration is the major source of calcium in solution since the amount of calcium coming from dissociation of the chelate is very small, especially in presence of Ca2+ left in solution. However, let us calculate the amount of calcium released from the chelate: mmol CaY2- formed = 2.5 [CaY2-] = 2.5/125 = 0.02 M
- 21. 21 Kf = [CaY2-]/[Ca2+][Y4-] 5x1010 = (0.02 – x)/((0.06 + x) * a4 x) assume that 0.02>>x x = 1.9x10-11 The assumption is valid even without verification. [Ca2+] = 0.06 + 1.9x10-11 = 0.06 M pCa = 1.22
- 22. 22 3. After addition of 50 mL EDTA mmol EDTA added = 0.10 x 50 = 5.0 mmol Ca2+ left = 10 – 5.0 = 5.0 [Ca2+]left = 5.0/150 = 0.033 M We will see by similar calculation as in step above that the amount of Ca2+ coming from dissociation of the chelate is exceedingly small as compared to amount left. However, for the sake of practice let us perform the calculation: mmol CaY2- formed = 5.0 [CaY2-] = 5.0/150 = 0.033 M
- 23. 23 Kf = [CaY2-]/[Ca2+][Y4-] 5x1010 = (0.033 – x)/((0.033 + x)* a4 x) assume that 0.033>>x x = 5.7x10-11 The assumption is valid even without verification. [Ca2+] = 0.033+ 5.7x10-11 = 0.033 M pCa = 1.48
- 24. 24 4. After addition of 100 mL EDTA mmol EDTA added = 0.10 x 100 = 10 mmol Ca2+ left = 10 – 10 = 0 This is the equivalence point. The only source for Ca2+ is the dissociation of the Chelate mmol CaY2- formed = 10 [CaY2-] = 10/200 = 0.05 M Ca2+ + Y4- D CaY2- kf = 5.0x1010
- 25. 25 Kf = [CaY2-]/[Ca2+][Y4-] 5x105 = (0.05 – x)/(x* a4 x) assume that 0.05>>x, x = 1.7x10-6 Relative error = (1.7x10-6/0.05) x 100 = 3.4x10-3% [Ca2+] = 1.7x10-6 M, pCa = 5.77 5. After addition of 150 mL EDTA mmol EDTA added = 0.10 x 150 = 15 mmol EDTA excess = 15 – 10 = 5.0 CT = 5.0/250 = 0.02 M mmol CaY2- = 10 [CaY2-] = 10/250 = 0.04 M Ca2+ + Y4- D CaY2- kf = 5.0x1010
- 26. 26 Kf = [CaY2-]/[Ca2+][Y4-] 5x1010 = (0.04 – x)/(x* a4(0.02 + x) ) assume that 0.02>>x x = 1.1x10-10 The assumption is valid [Ca2+] = 1.1x10-10 M pCa = 9.95
- 27. 27 6. After addition of 200 mL EDTA mmol EDTA added = 0.10 x 200 = 20 mmol EDTA excess = 20 – 10 = 10 CT = 10/300 = 0.033 M mmol CaY2- = 10 [CaY2-] = 10/300 = 0.033 M Ca2+ + Y4- D CaY2- kf = 5.0x1010
- 28. 28 Kf = [CaY2-]/[Ca2+][Y4-] 5x1010 = (0.033 – x)/(x* a4(0.033 + x) ) assume that 0.033>>x x = 5.7x10-11 The assumption is undoubtedly valid [Ca2+] = 5.7 x10-11 M pCa = 10.24