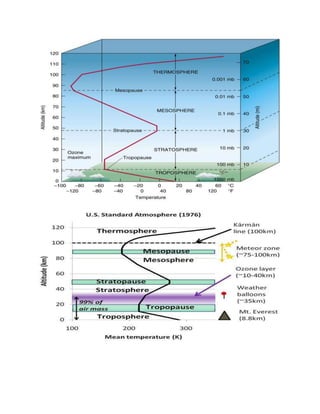
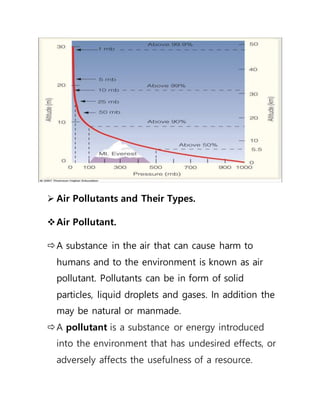
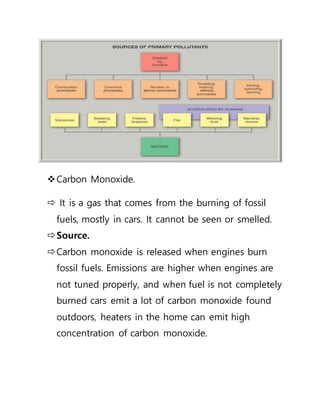
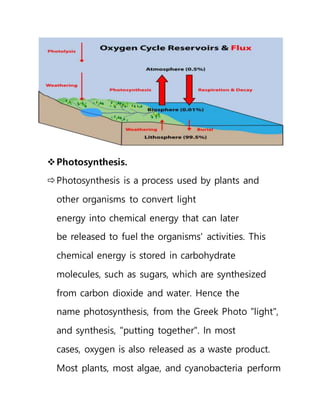
This document provides an overview of atmospheric pollution and the structure and composition of the atmosphere. It discusses the different sources of air pollution, including mobile, stationary, area, and natural sources. It then describes the layers of the atmosphere from troposphere to exosphere, including details on temperature and pressure profiles. Key components like the ozone layer and ionosphere are explained. Processes such as photosynthesis, photolysis, and photoionization are also summarized.