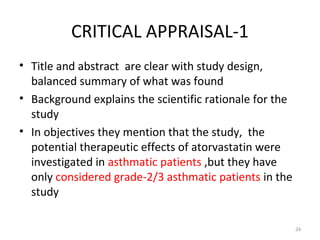
This study evaluated the effects of atorvastatin for treating asthma patients through a double-blind randomized clinical trial. 62 patients with mild to moderate persistent asthma were alternately allocated to receive either atorvastatin or a placebo for 8 weeks. Asthma control was assessed before and after treatment using the Asthma Control Test questionnaire. The study found no significant differences in asthma control between the atorvastatin and placebo groups, indicating that atorvastatin was not effective for treating mild to moderate asthma over the study period based on the measures used. The summary discusses some limitations of the study related to sensitivity of outcome measures and potential bias from the alternating allocation method used.