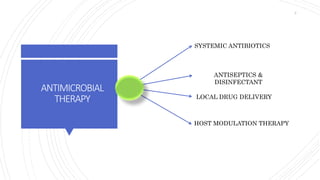
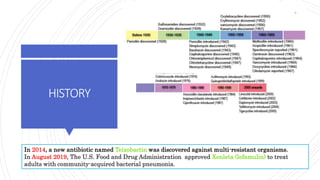
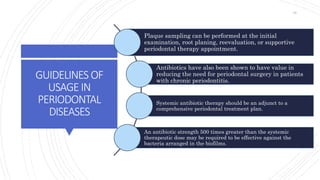
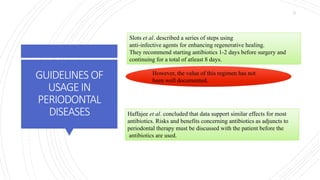
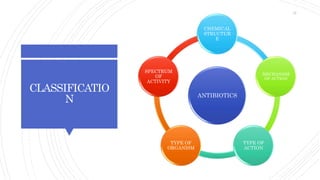
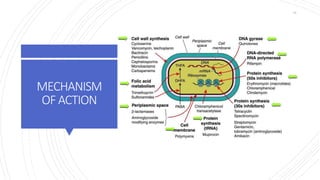
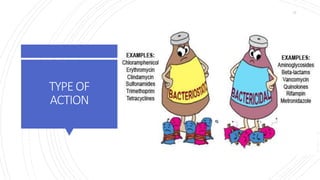
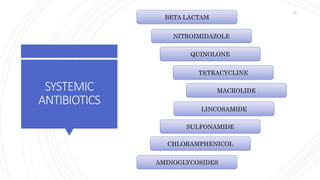
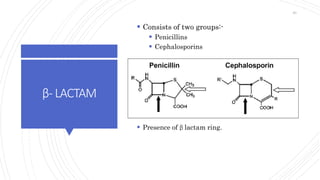
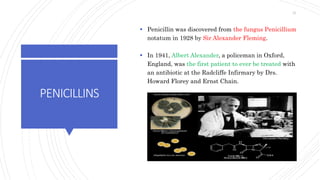
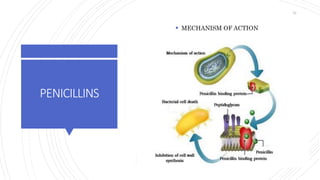
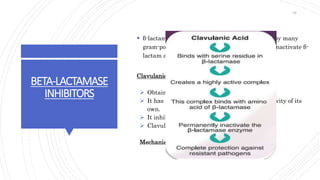
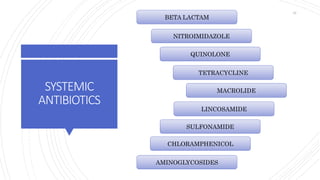
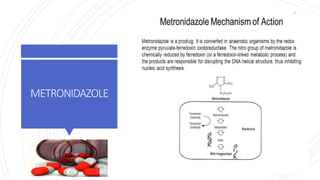
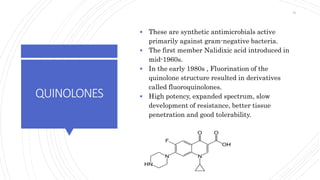
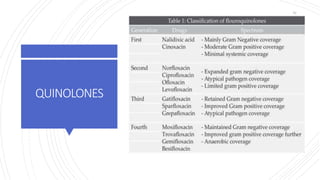
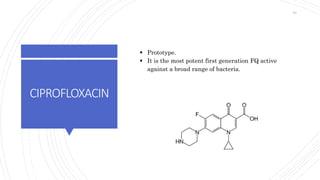
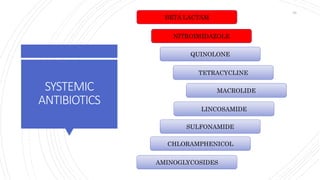
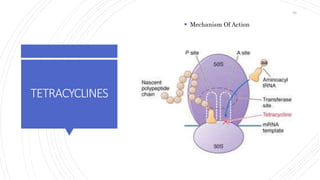
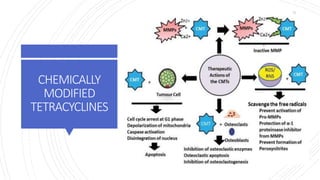
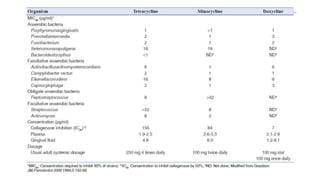
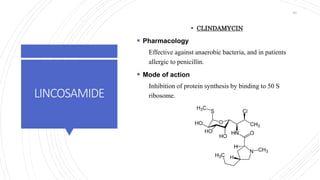
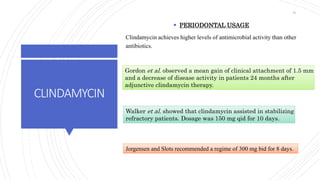
The document discusses antimicrobial therapy in periodontics. It provides an overview of the history and rationale for using antibiotics in periodontal therapy. It classifies antibiotics based on their chemical structure, mechanism of action, type of action, types of organisms affected, and spectrum of activity. Specific antibiotics discussed in detail include beta-lactams like penicillins and cephalosporins. Guidelines for use of antibiotics in periodontal diseases are presented.