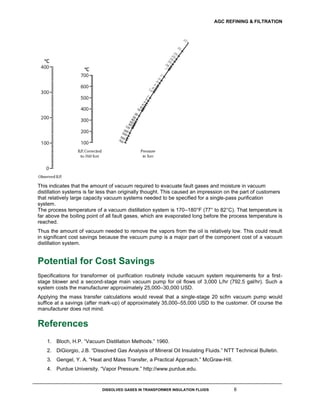
This document discusses dissolved gases in transformer insulation fluids. It explains that insulation fluid inside transformers breaks down over time, generating gases that can indicate electrical faults. The types and amounts of gases produced correspond to the severity of the fault. Common fault gases are identified. The document also discusses how vacuum distillation can be used to remove dissolved gases and moisture from insulation fluids in a cost-effective manner, noting that lower vacuum levels than typically specified are sufficient given the low boiling points of fault gases. This could enable significant cost savings for purification systems.