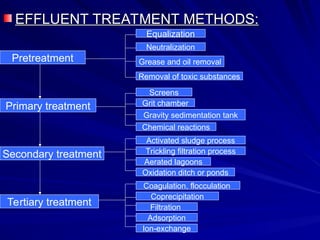
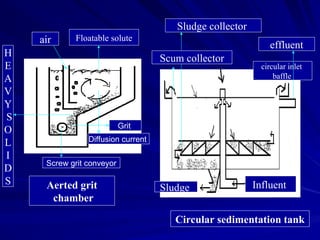
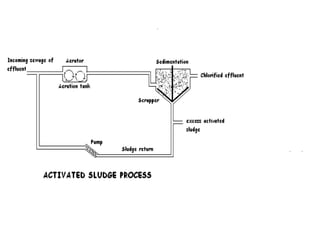
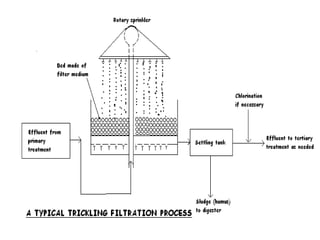
Effluent treatment is the recycling of industrial wastewater to make it reusable, aimed at preventing environmental harm and recovering valuable materials. The document details various effluent treatment methods, tests for contaminants, and procedures for assessing the quality of wastewater, including measures of toxic metals, pH, and oxygen demand. Effective treatment minimizes the adverse effects on natural water bodies and ensures compliance with environmental standards.