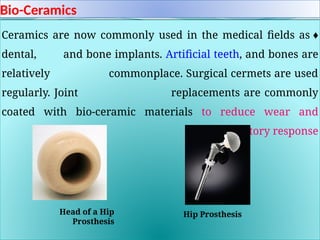
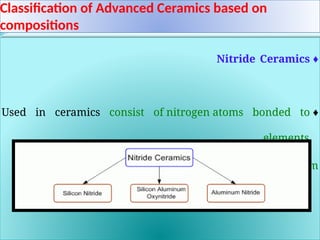
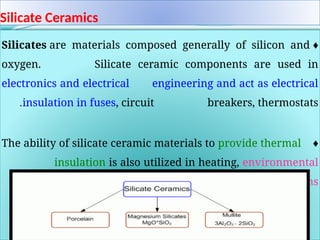
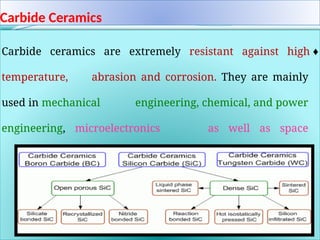
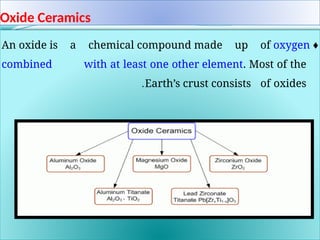
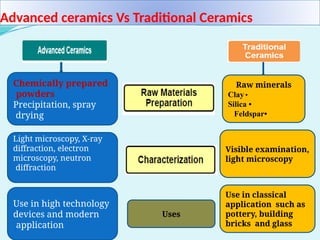
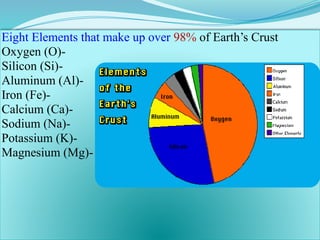
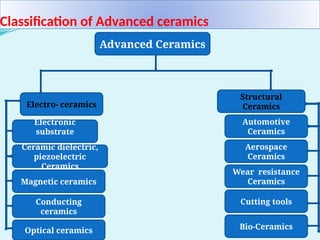
The document discusses advanced ceramics and their classifications, highlighting their industrial applications and the unique properties that make them suitable for various uses, such as in electronics and aerospace. It covers types of advanced ceramics, including conducting, dielectric, piezoelectric, and bio-ceramics, detailing their specific characteristics and manufacturing challenges. Additionally, the text contrasts advanced ceramics with traditional ceramics, emphasizing differences in composition and applications.



![Piezoelectric ceramics
♦
Piezoelectricity is a property of certain classes of crystalline
materials including Barium Titanate BaTiO3 and Lead
Zirconate Titanates Pb[ZrxTi1-x]O3(PZT)
.
.
♦
When mechanical pressure is applied to one of these materials,
the crystalline structure produces a voltage proportional to
the pressure. Conversely, when an electric field is applied, the
structure changes shape producing dimensional changes in
the material
.
♦
Use as sensors , actuators and transducers
.](https://image.slidesharecdn.com/advancedceramics-240821192548-2e02c0f1/85/Advanced-Ceramics-for-different-applications-pptx-7-320.jpg)
![Magnetic ceramics
♦
Magnetic ceramics are made of ferrites, which are
crystalline minerals composed of iron oxide in
combination with some other metal. They are given
the general chemical formula M(FexOy), M
representing other metallic elements than iron. The
most familiar ferrite is magnetite, a naturally occurring
ferrous ferrite (Fe[Fe2O4], or Fe3O4) commonly known as
lodestone
.
Ceramic Magnets](https://image.slidesharecdn.com/advancedceramics-240821192548-2e02c0f1/85/Advanced-Ceramics-for-different-applications-pptx-8-320.jpg)