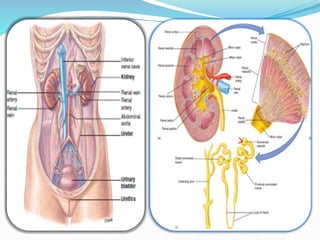
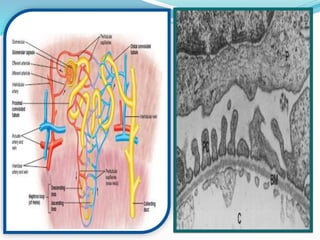
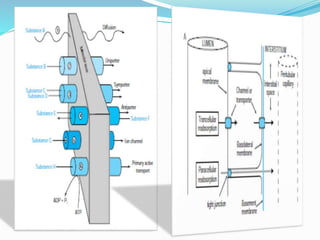
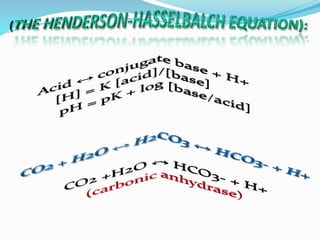
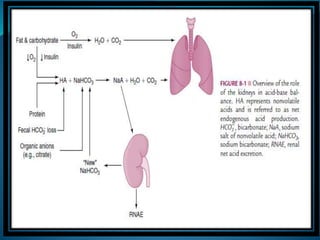
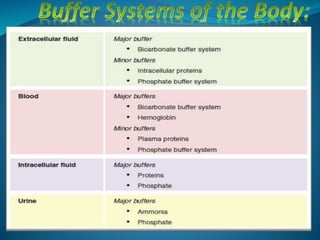
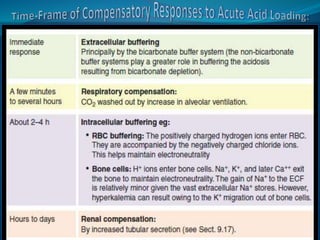
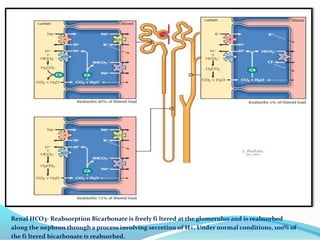
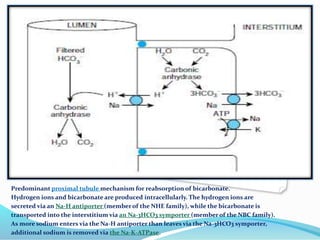
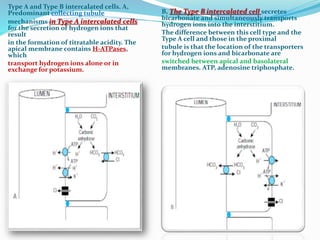
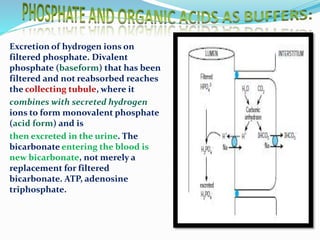
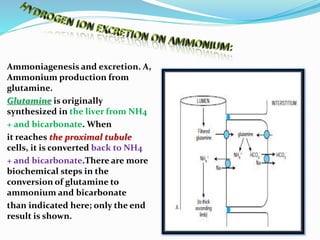
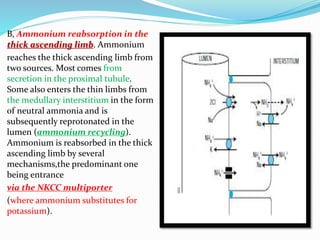
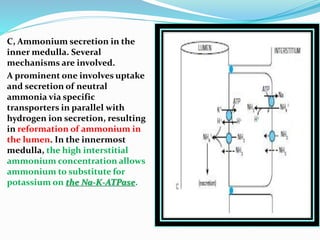
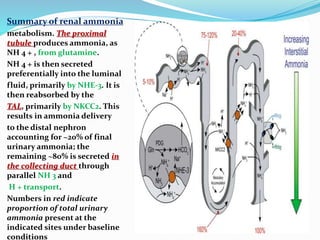
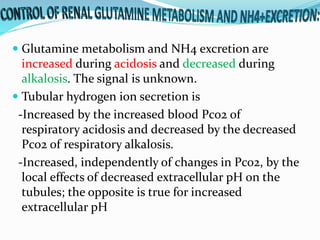
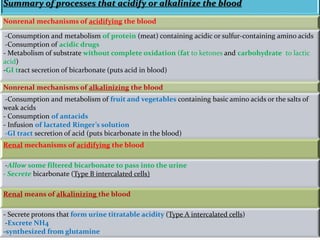
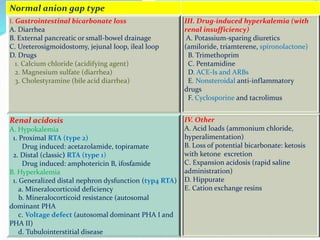
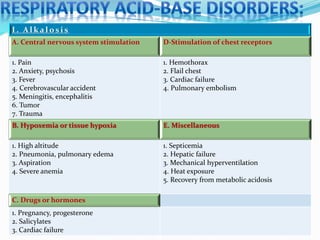
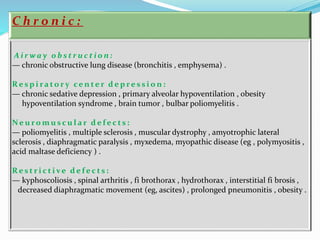
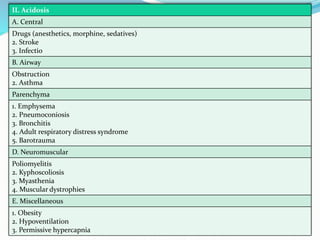
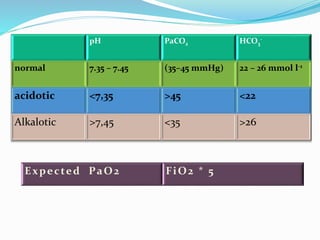
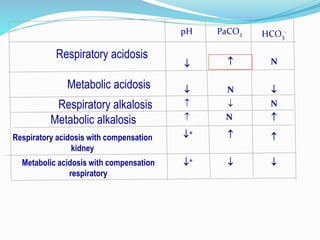
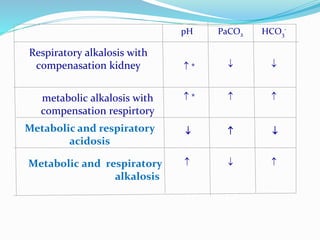
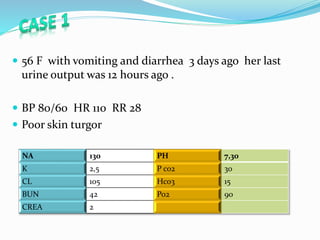
This document discusses acid-base balance and the renal regulation of acid-base balance. It provides guidelines for how acids and bases obey the balance principle and how body fluids are buffered. It describes how the input and output of acids can alter bicarbonate levels but not carbon dioxide partial pressure. It explains the independent excretion of carbon dioxide and bicarbonate. It also details the roles of the kidney in regulating acid-base balance through reabsorption and secretion in different tubular segments and production of ammonium ions.