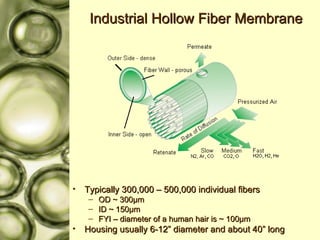
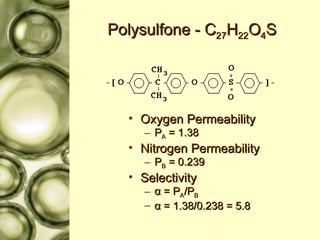
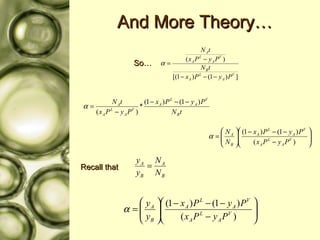
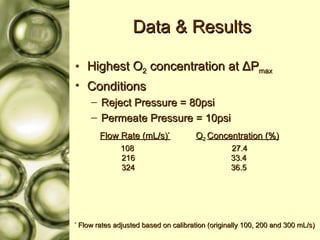
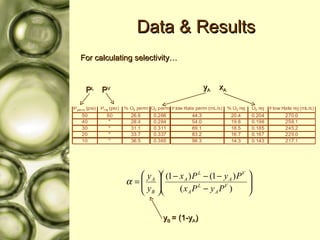
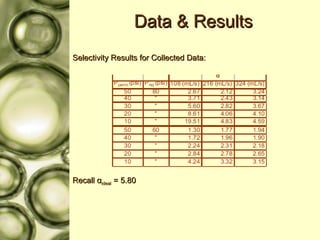
The document discusses gas membrane separation using hollow fiber membranes to separate an air stream into enriched oxygen and nitrogen streams. An experiment was conducted to determine the optimal conditions for separation, with the highest oxygen concentration achieved at the largest pressure difference and higher flow rates. The results showed that the experimental selectivity increased with the pressure difference but was slightly lower than the ideal selectivity value.