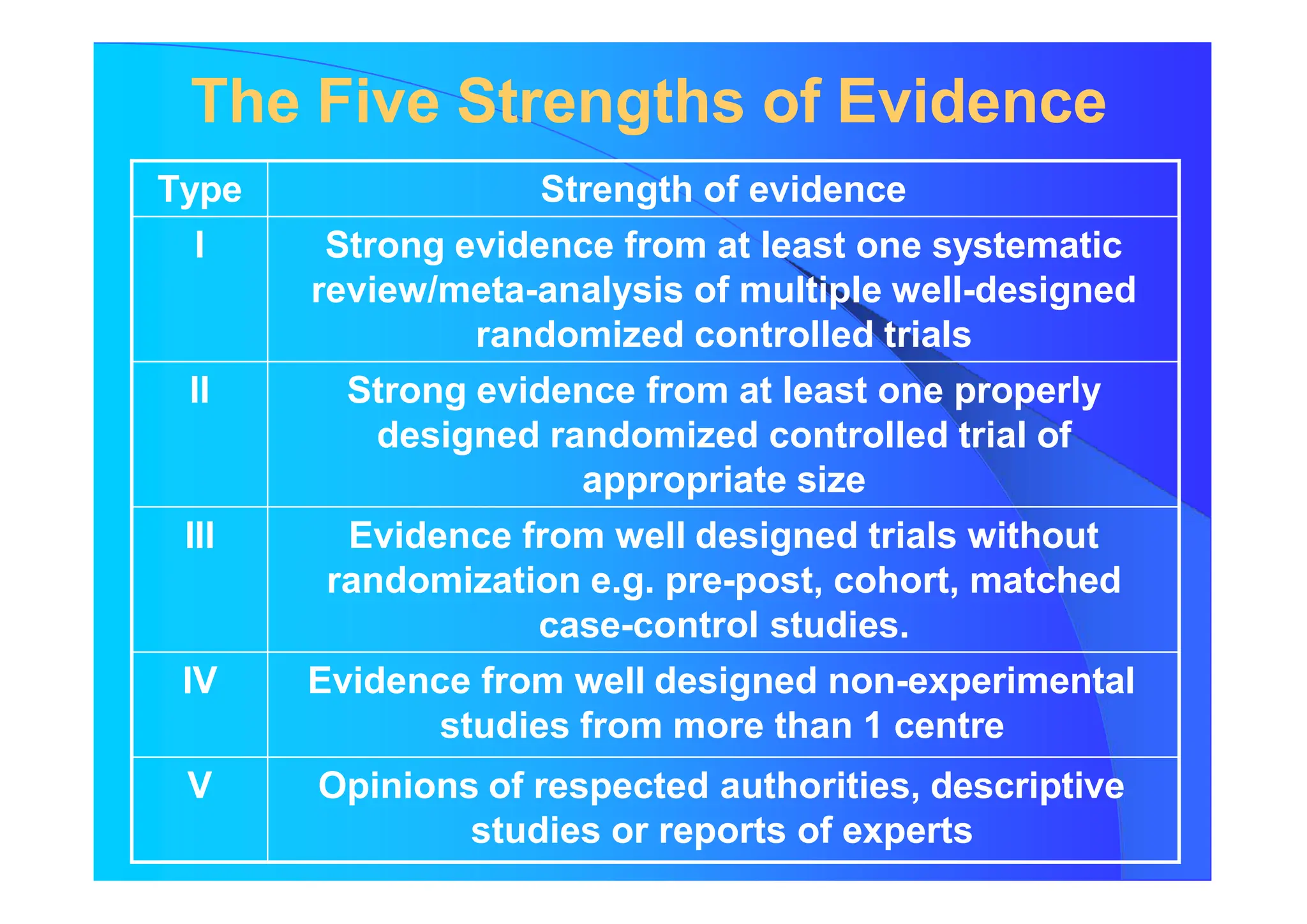
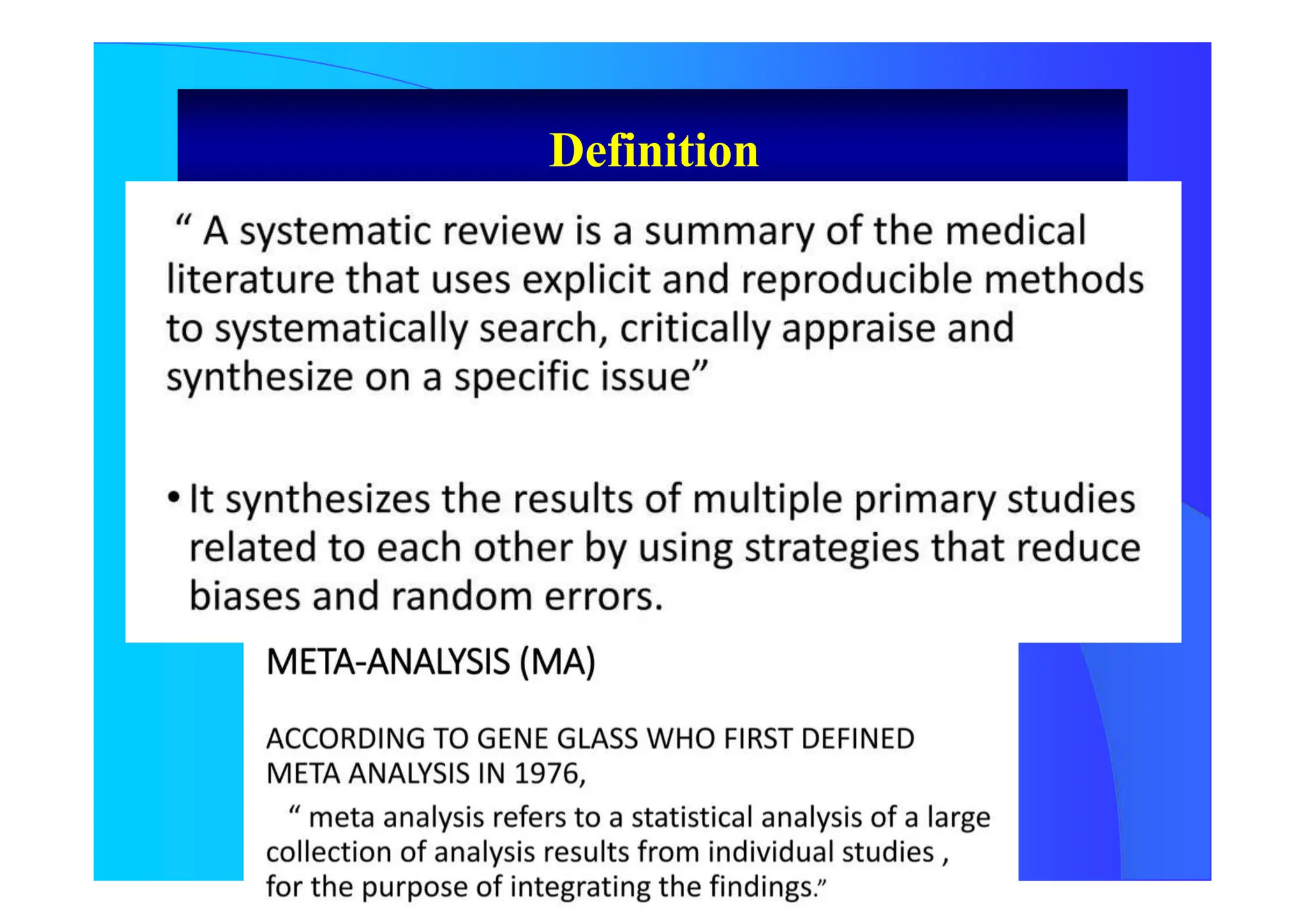
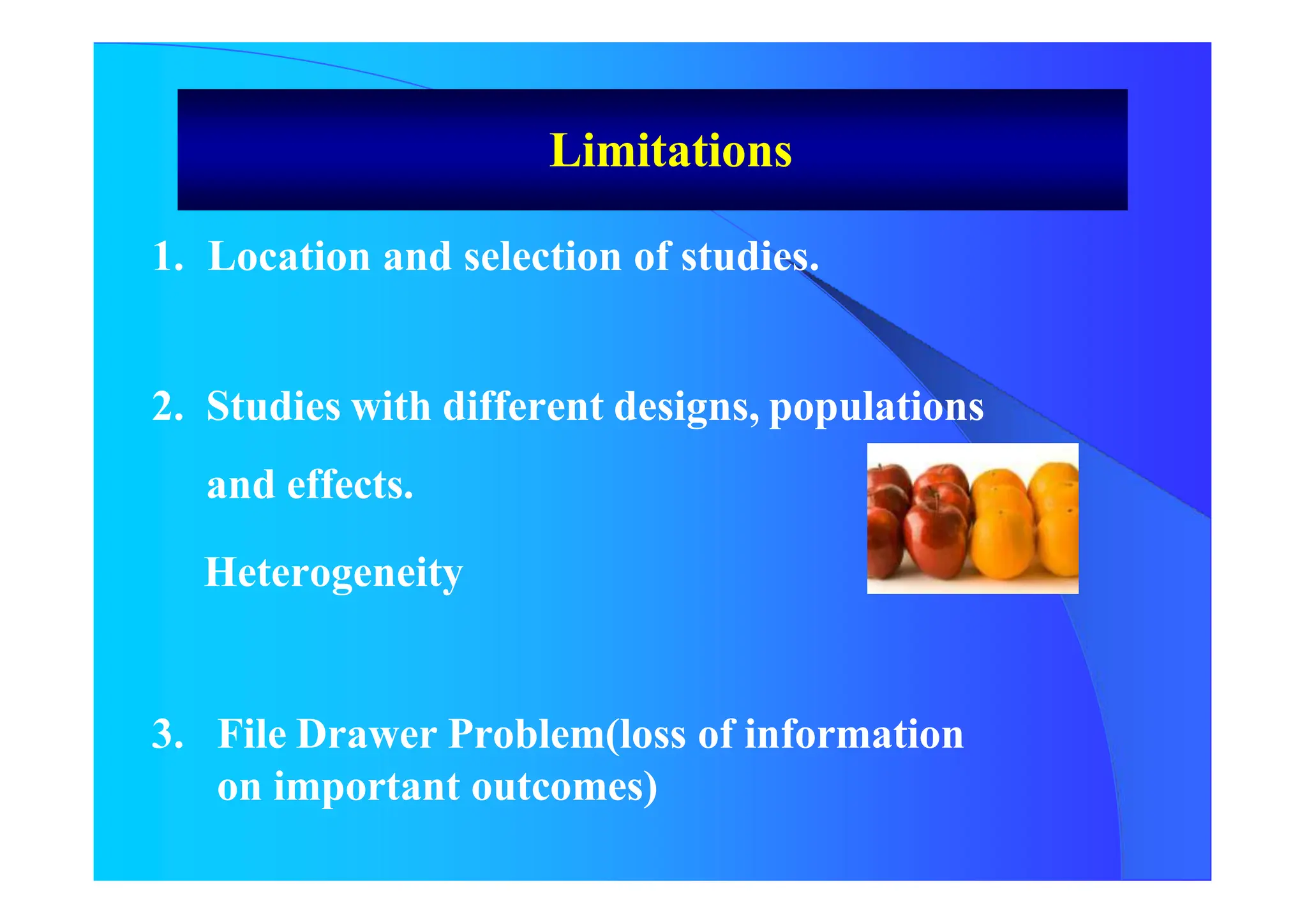
The document discusses the methodology and advantages of meta-analysis and systematic reviews, emphasizing the importance of evidence strength and types. It details various phases of conducting meta-analyses, including data collection, pooling results using different models, and addressing limitations such as potential biases. Key references and statistical techniques used in these analyses are also highlighted.





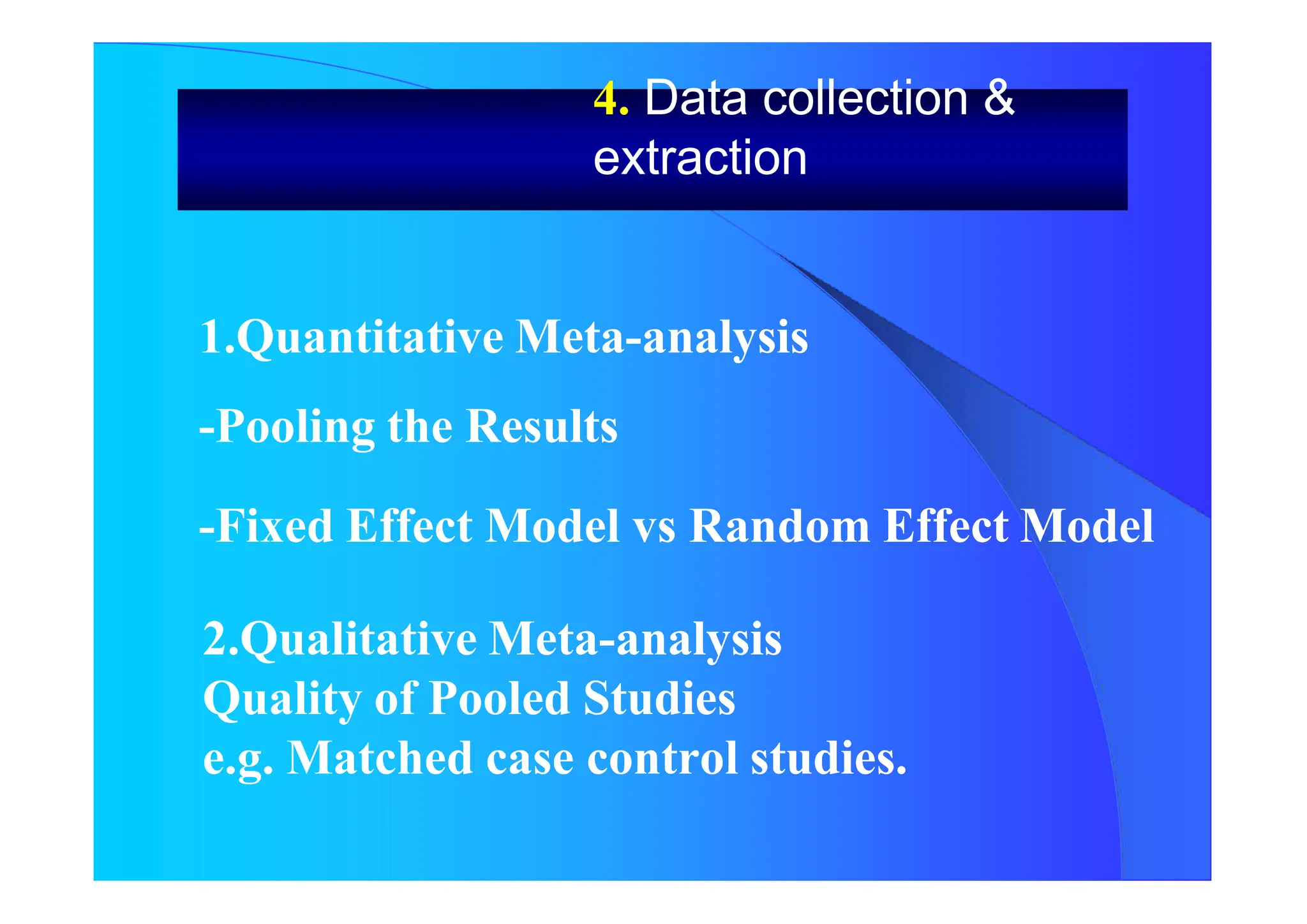
![. Quantitative Meta-analysis
- Combing the Results
= [ pi i ] / pi
pi Weight of every Study
i Parameter selected for investigation
Phases](https://image.slidesharecdn.com/9-240525090115-225b4bcc/75/9-Meta-analysis-Systematic-Review-summary-view-pptx-12-2048.jpg)

![1. Mantel Hanzel
Pooling
E x p o s e d D i s e a s e
P r e s e n t
D i s e a s e
A b s e n t
T o t a l
P r e s e n t A B A + B
A b s e n t C D C + D
T o t a l A + C B + D N
RRMH = [ (ci bi / ni ) (ai di / bi ci ) ] / (ci bi / ni )
Var RR = RRi [ 1/a + 1/b + 1/c + 1/d ]
RRMH 95% CI = RRMH x EXP [ + 1,96 Var RR (In RRMH)]](https://image.slidesharecdn.com/9-240525090115-225b4bcc/75/9-Meta-analysis-Systematic-Review-summary-view-pptx-16-2048.jpg)
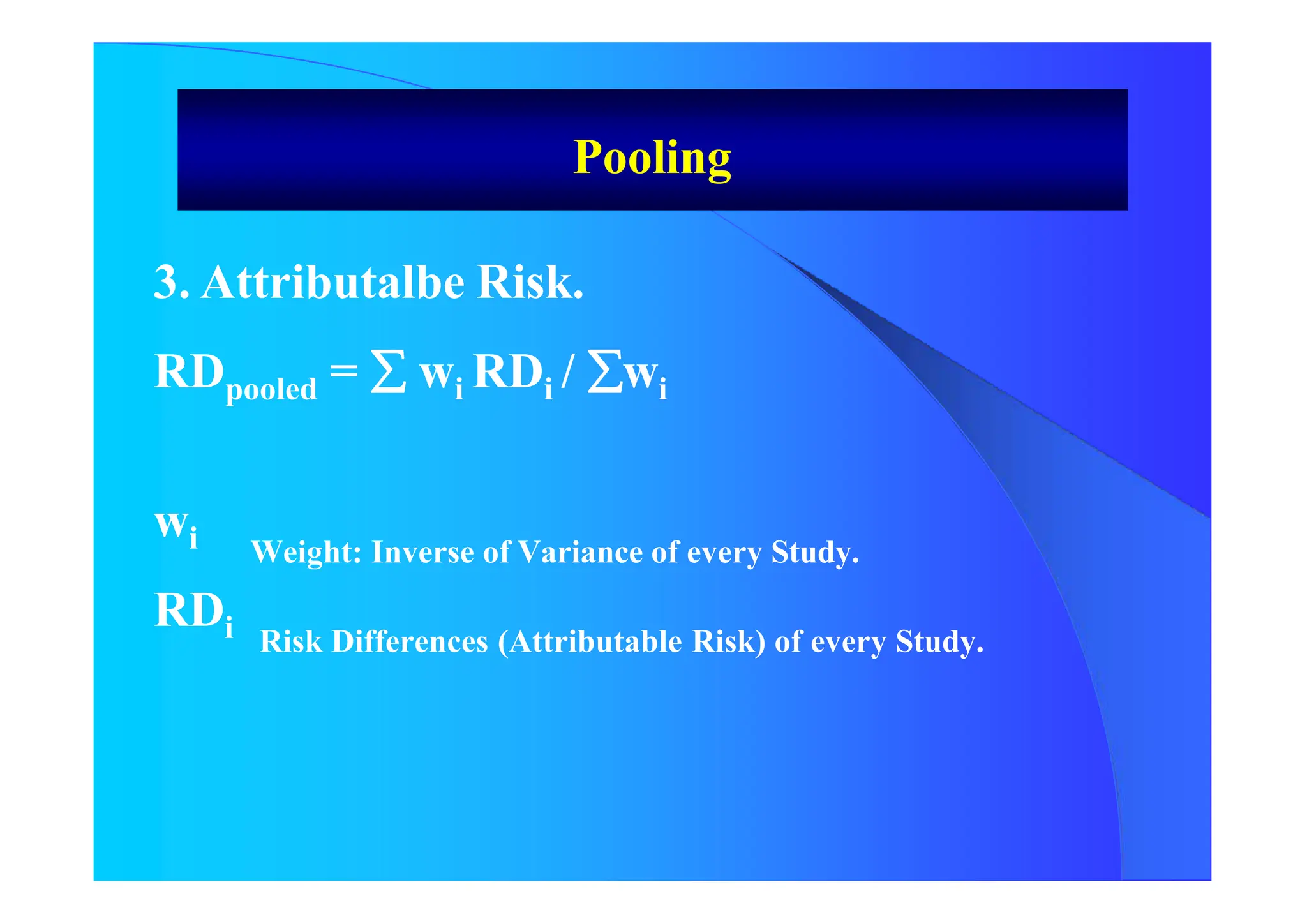
![Pooling
2. Inverse of Variance
RRW = Exp [ wi In RRi ] / wi
RRW Global Relative Risk.
wi Weight of every Study: Inverse of Variance of RR.
RRi Relative Risk of every Study
RR refers to RR, SMR, OR or POR
RRW 95% CI = In RRW * Exp [ + 1,96 Var (In RRw)]](https://image.slidesharecdn.com/9-240525090115-225b4bcc/75/9-Meta-analysis-Systematic-Review-summary-view-pptx-17-2048.jpg)

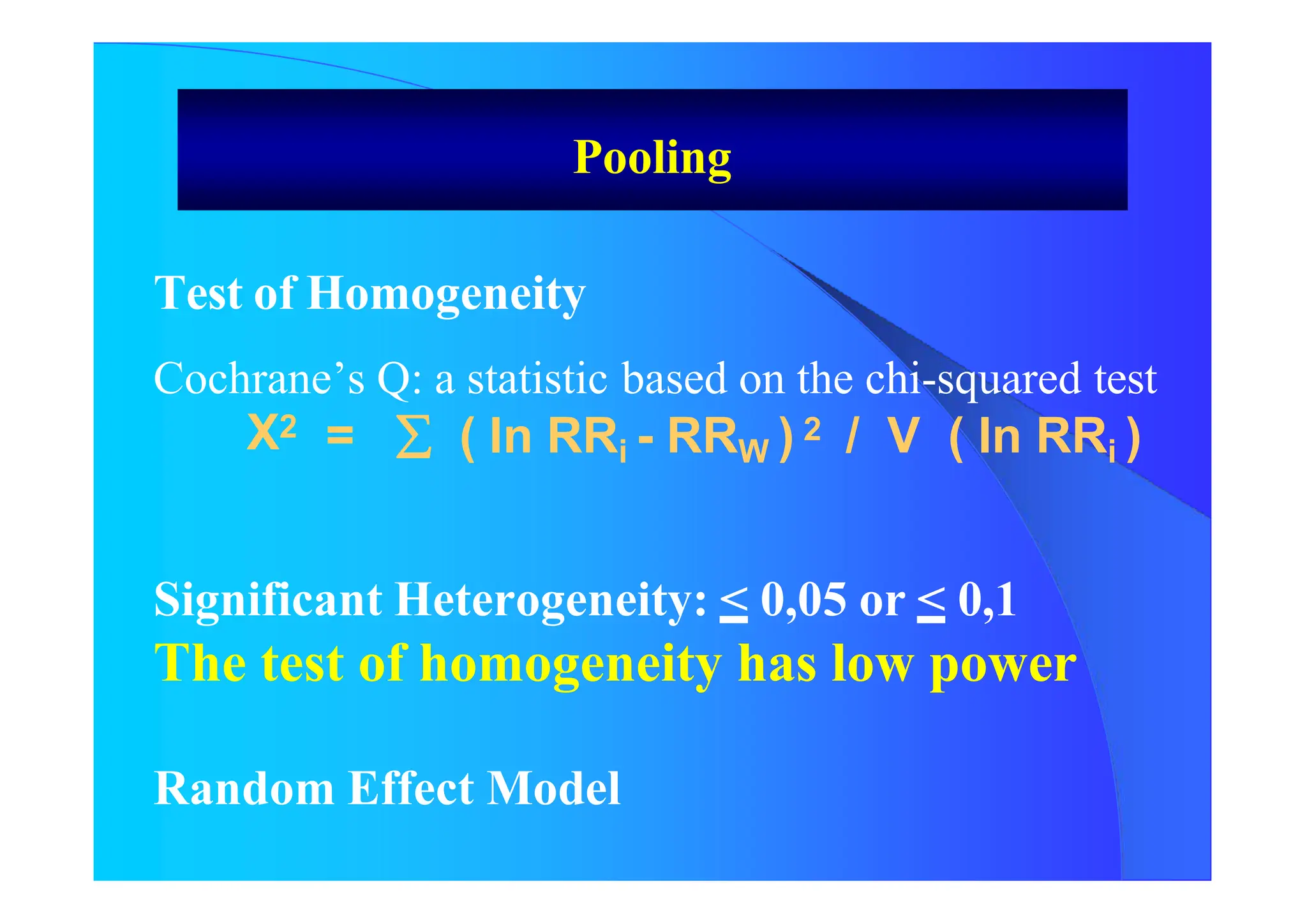
![Random Effect Model
RRA = Exp [ w*i In RRi ] / w*i
W*I will be calculated as follow:
W *i = 1/ [ V (In ORi) + VAR]
VAR = [Chi Square – (N-1)] / U
U = (N – 1) [WM - { var (w) / N* WM}]
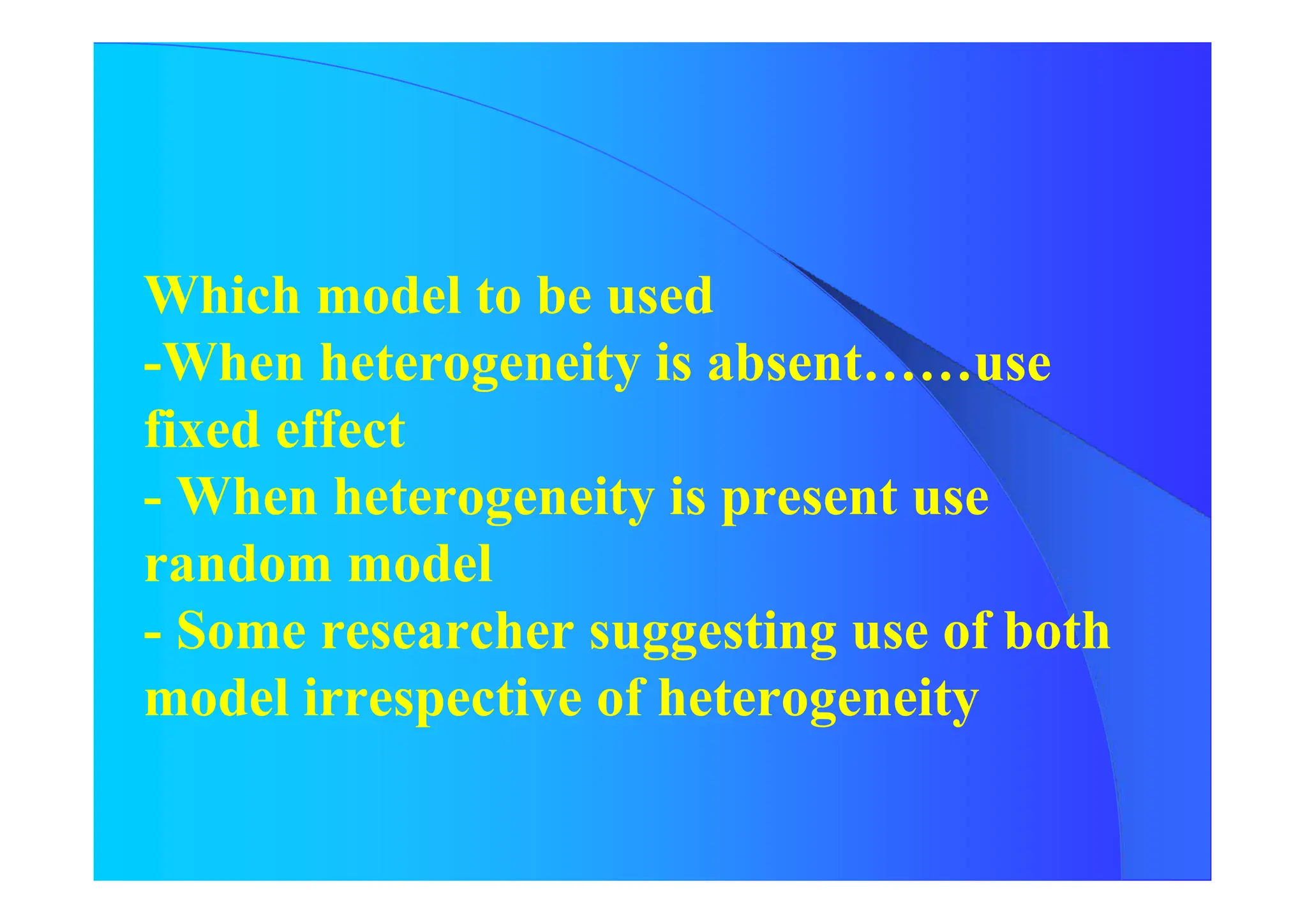
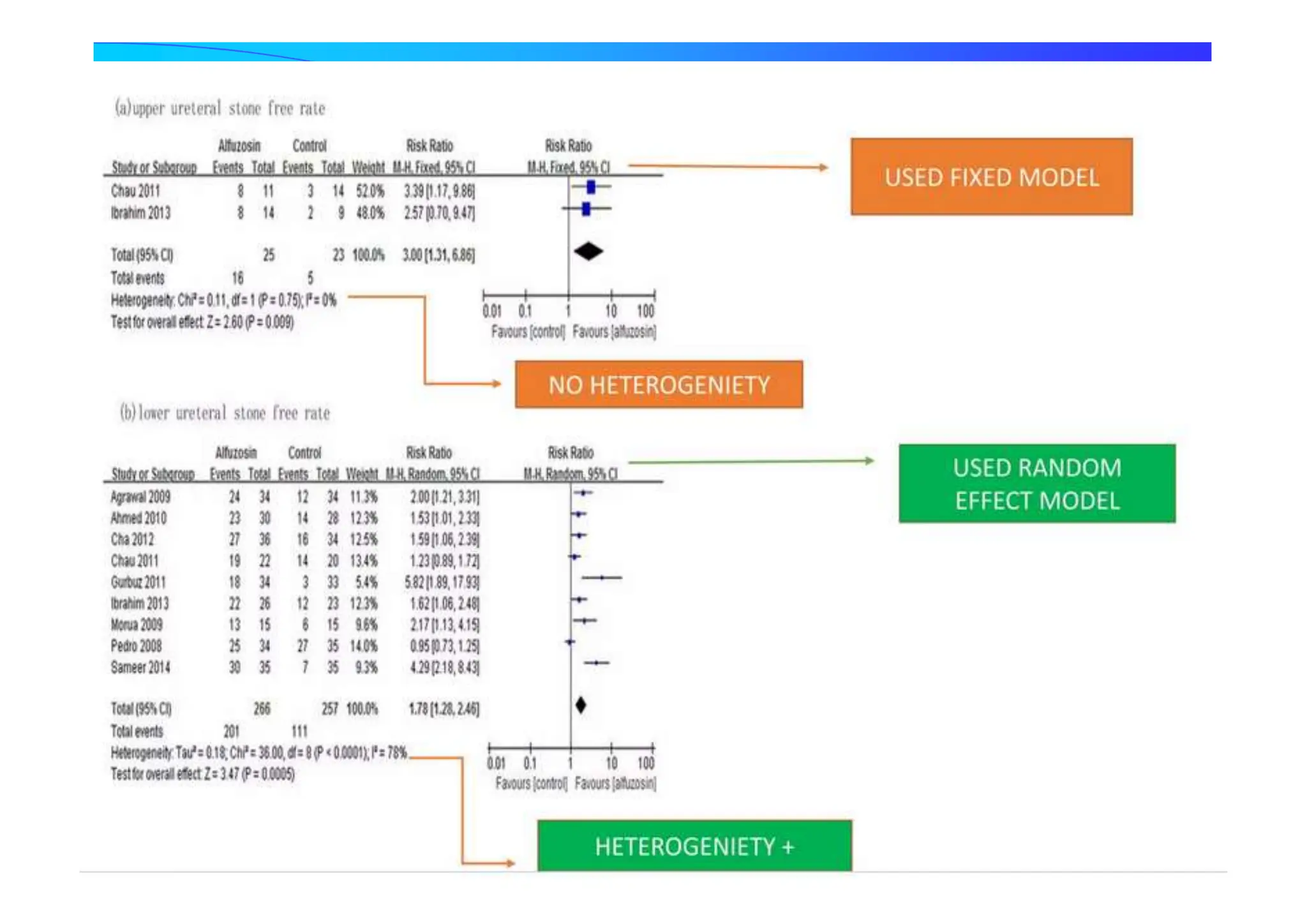
Results of both Fixed Effect Model and
Random Effect Model
Pooling](https://image.slidesharecdn.com/9-240525090115-225b4bcc/75/9-Meta-analysis-Systematic-Review-summary-view-pptx-23-2048.jpg)

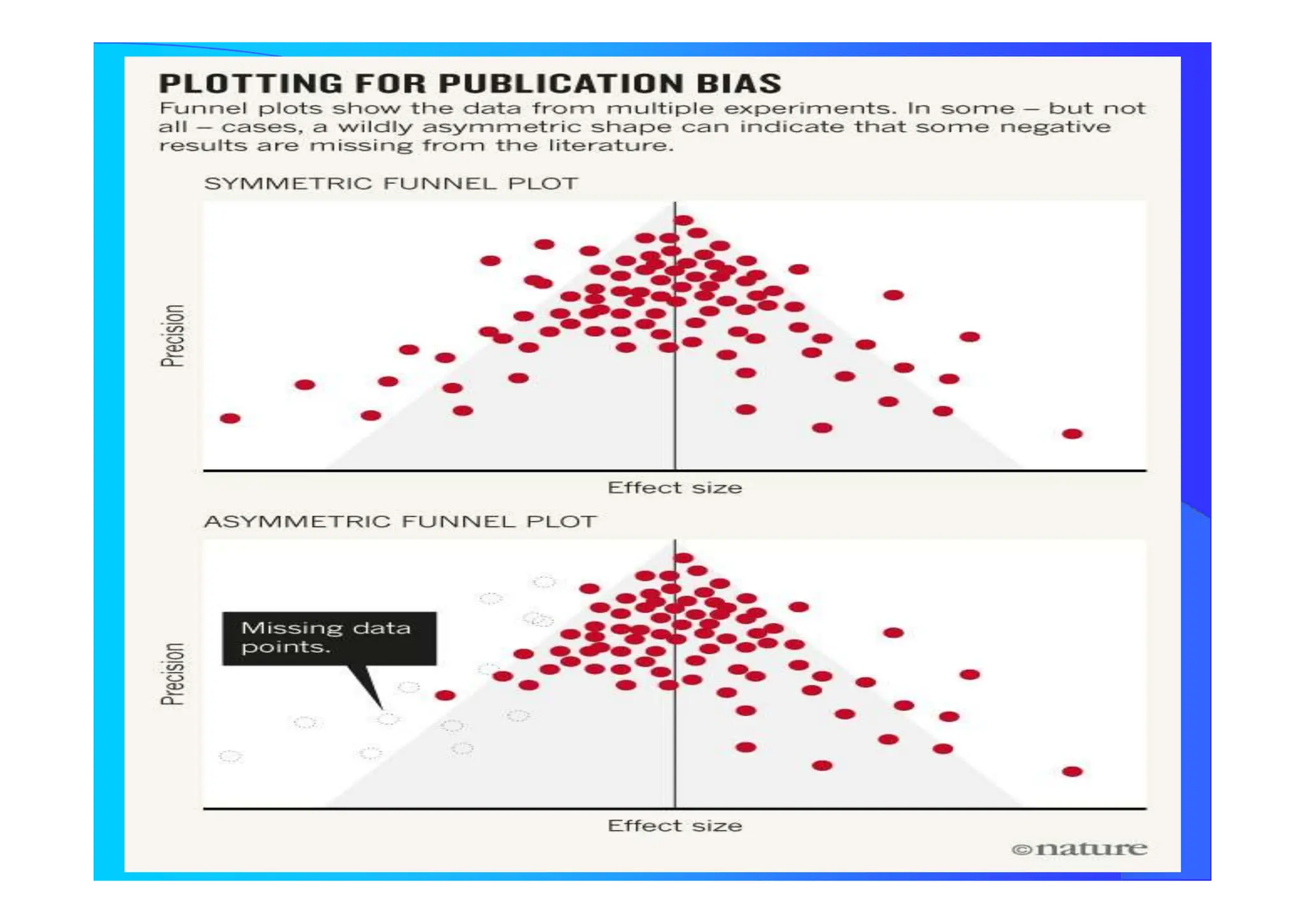
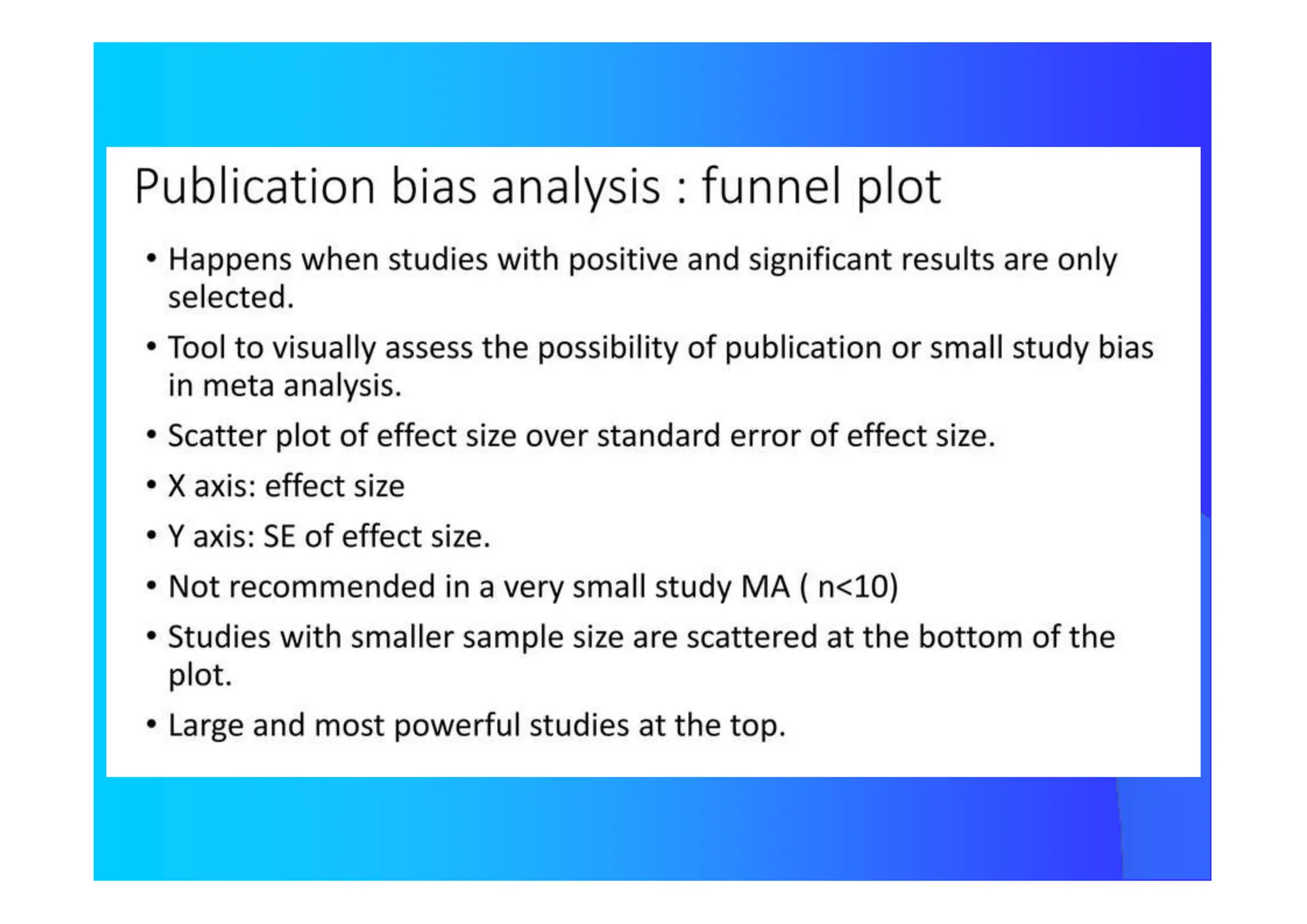
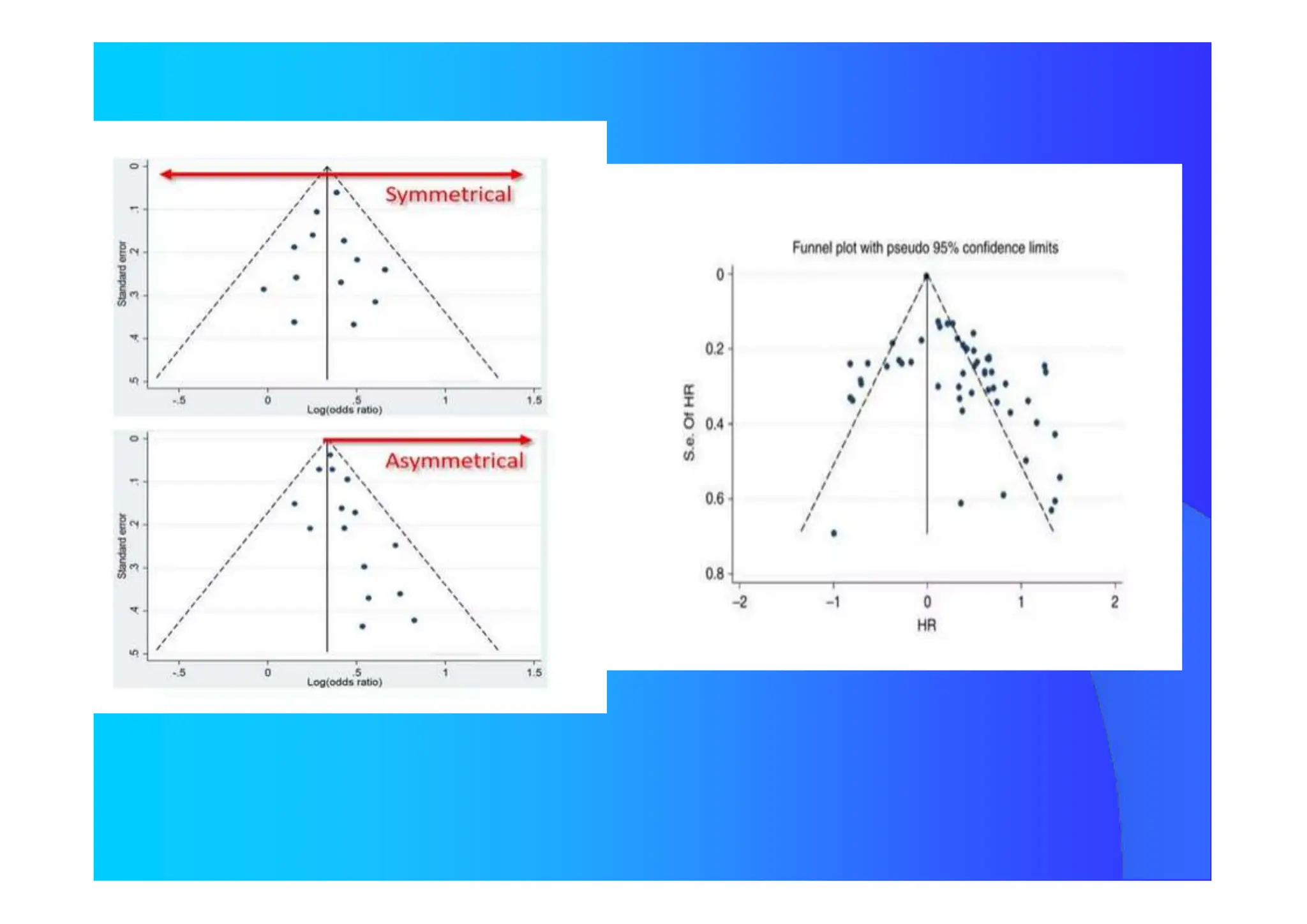
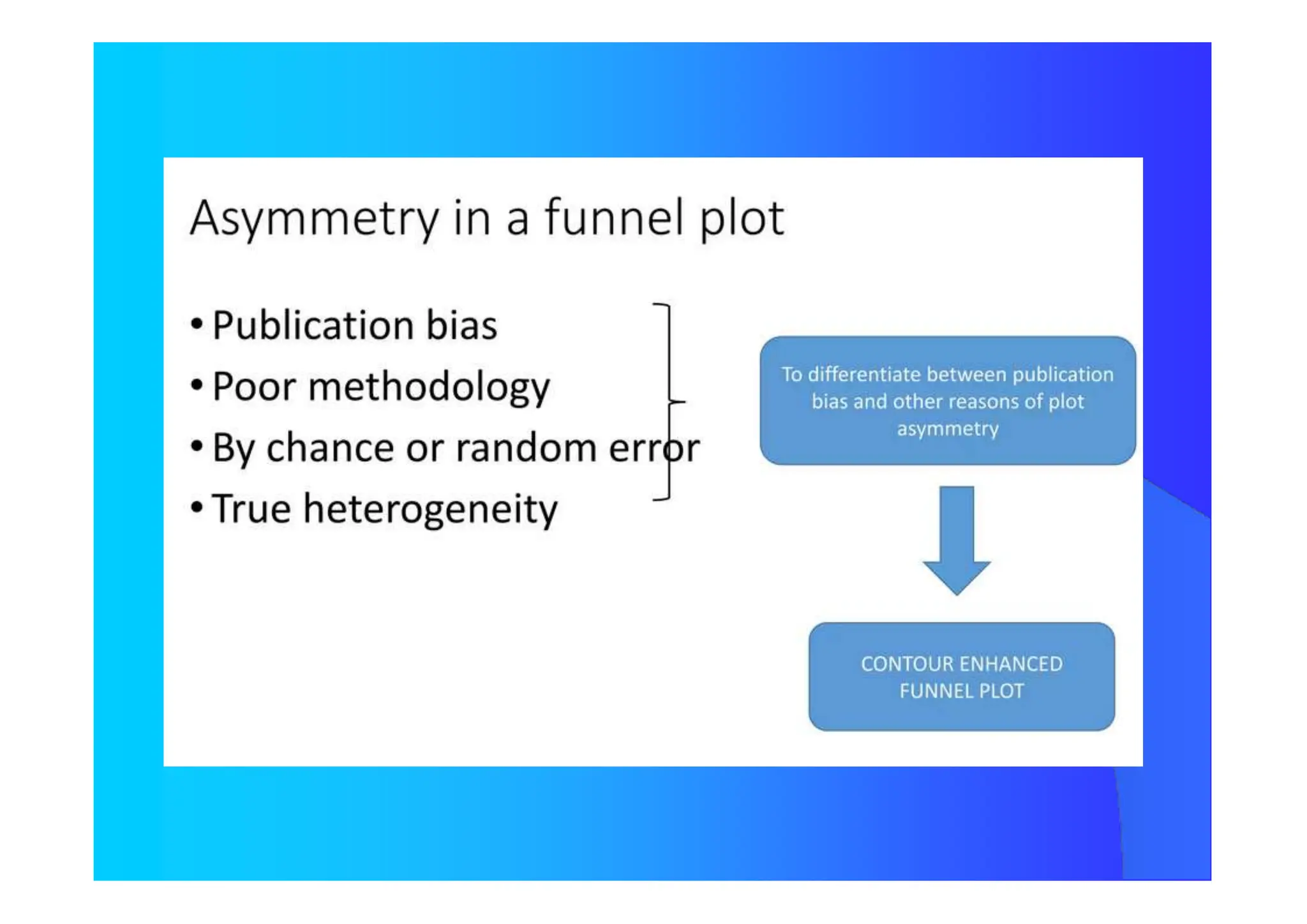
![How Many Studies Could Change The Results
ToAccept The Null Hypothesis?
Tolerance Index
N = [ K {K(RRW) 2 – 2,706} ] / 2,706
N Number of Studies not considerded and
with Null Results
K Number of Studies Included in the Review
Publication Bias](https://image.slidesharecdn.com/9-240525090115-225b4bcc/75/9-Meta-analysis-Systematic-Review-summary-view-pptx-29-2048.jpg)