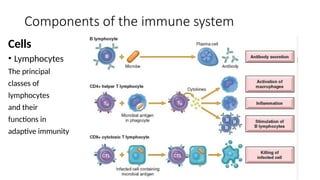
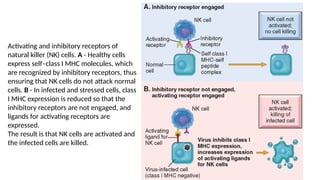
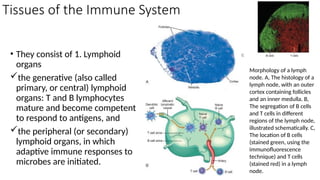
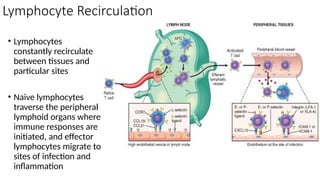
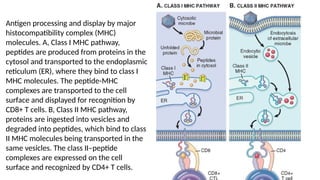
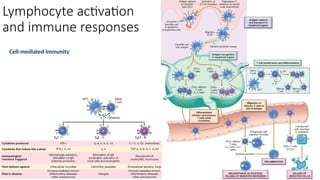
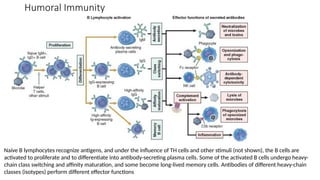
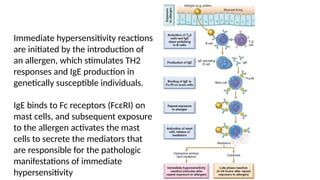
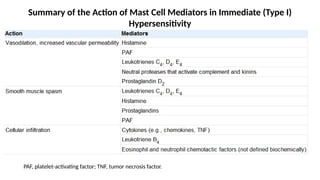
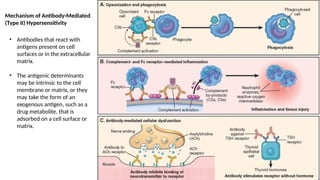
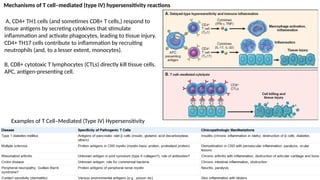
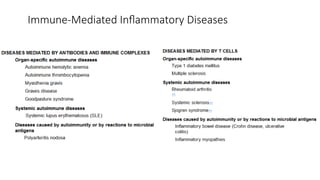
The document describes the immune response, outlining the two major types: innate immunity, which is a pre-existing defense against pathogens, and adaptive immunity, which develops and adapts to recognize specific antigens. It details various immune cells and their roles, including lymphocytes, macrophages, and natural killer cells, as well as the mechanisms of hypersensitivity and autoimmune disorders. Additionally, it explains the importance of immunological tolerance and the processes of central and peripheral tolerance to prevent harmful autoimmune reactions.