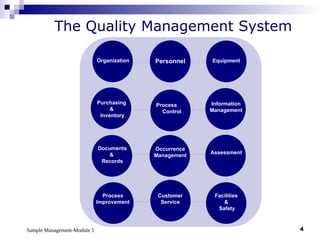
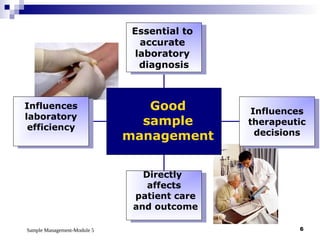
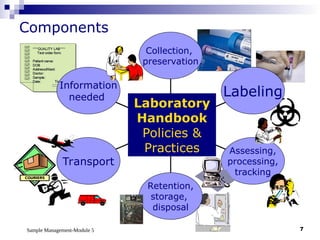
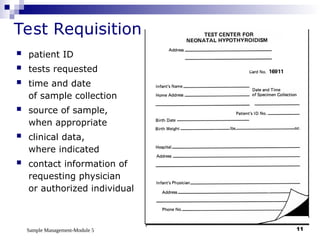
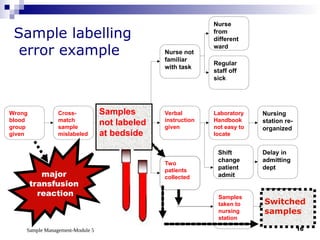
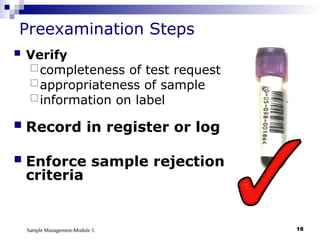
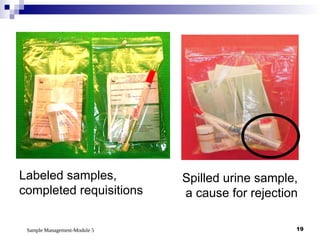
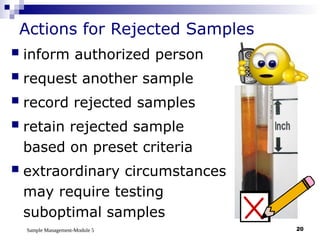
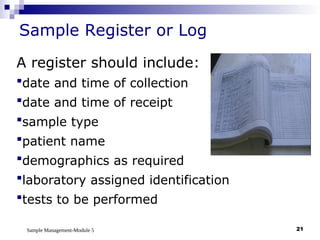
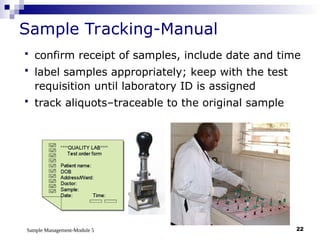
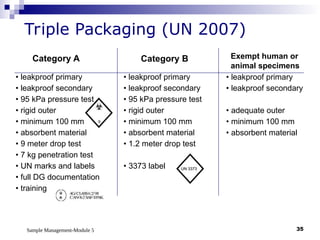
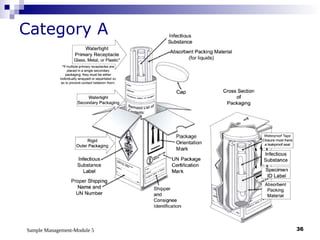
Module 5 focuses on sample management in laboratory settings, emphasizing the importance of proper collection, handling, storage, transport, and disposal of samples to ensure accurate examination results. Key learning objectives include recognizing sample collection errors, establishing a laboratory handbook for staff, and implementing a labeling and tracking system. Proper sample management significantly impacts patient care, testing accuracy, and diagnostic outcomes.