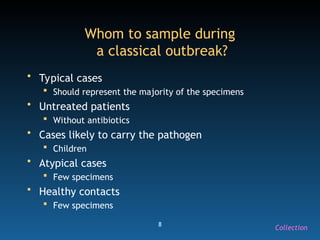
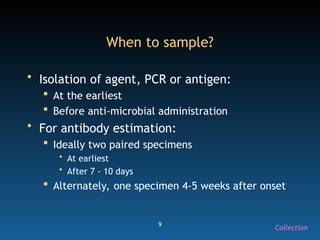
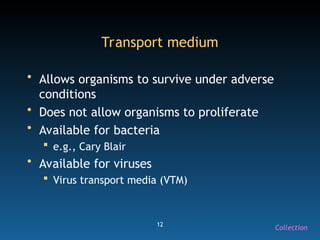
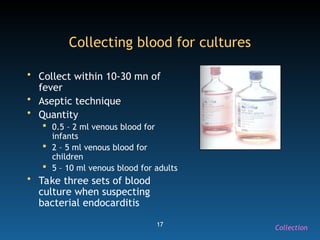
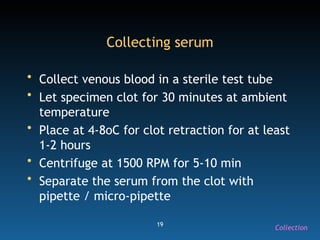
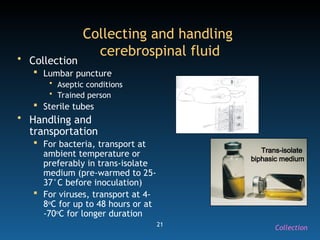
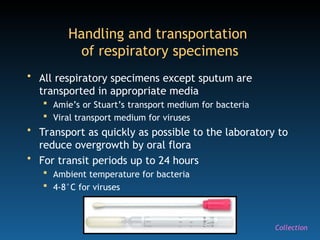
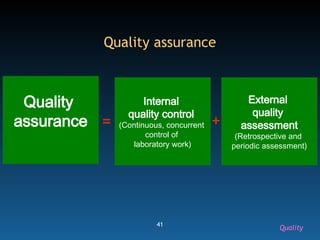
The document discusses the essential protocols for collaborating with laboratories during outbreak investigations, highlighting the importance of communication, specimen collection, and biosafety. It provides guidelines on specimen handling, transportation methods, and quality assurance measures to ensure accurate results and safety for all involved. Key recommendations include developing good rapport with labs, adhering to collection guidelines, and maintaining high biosafety standards.