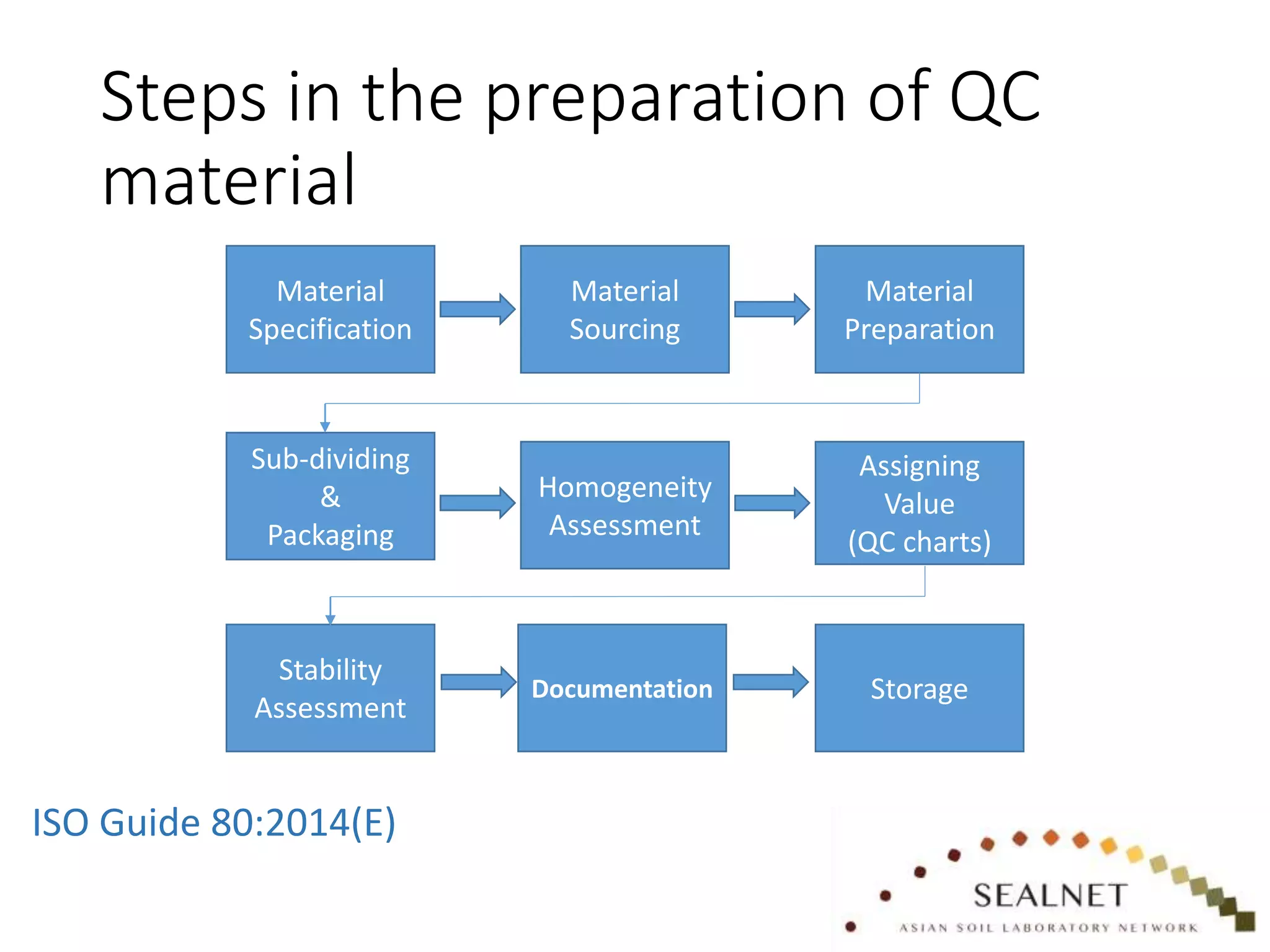
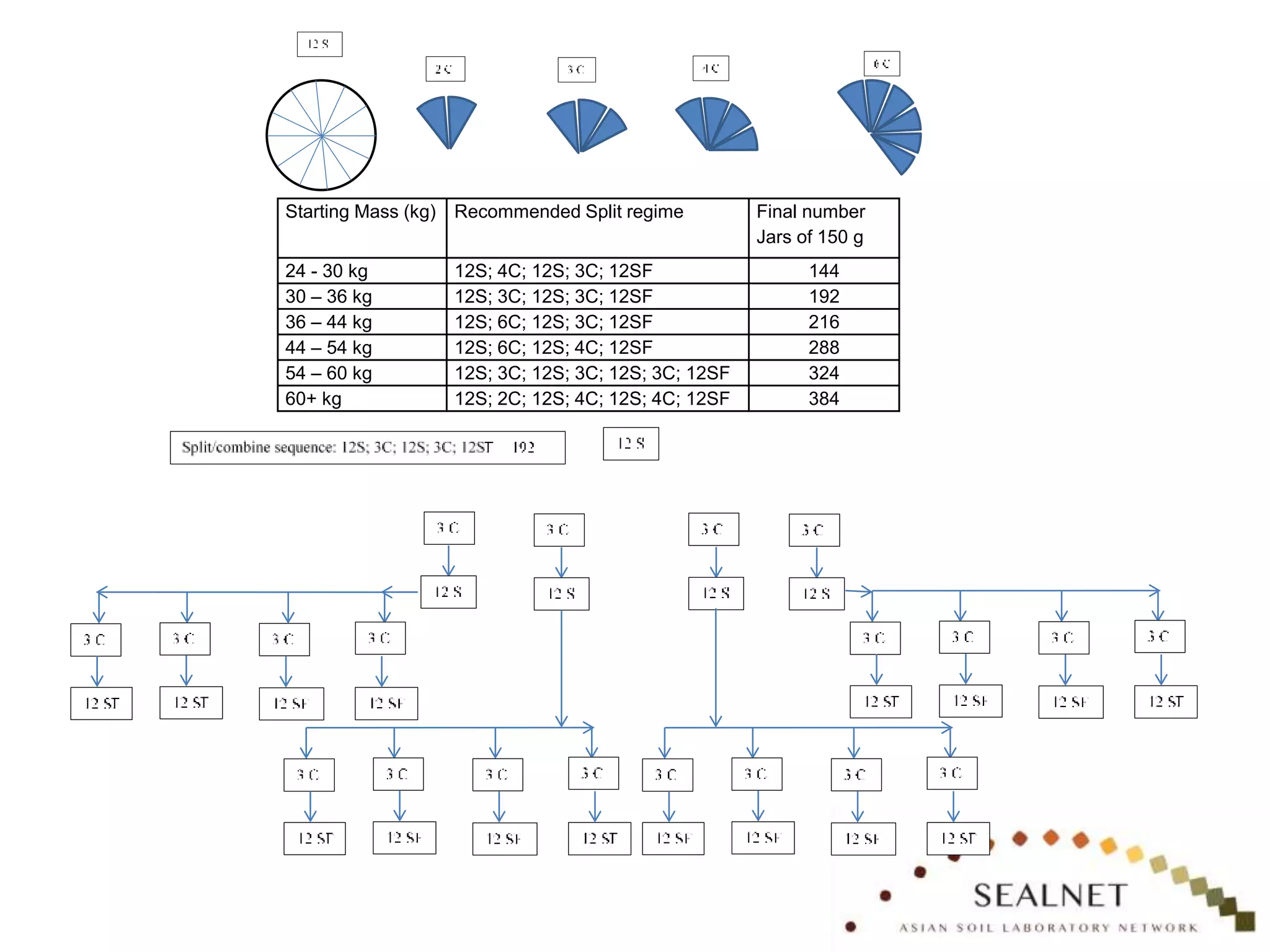
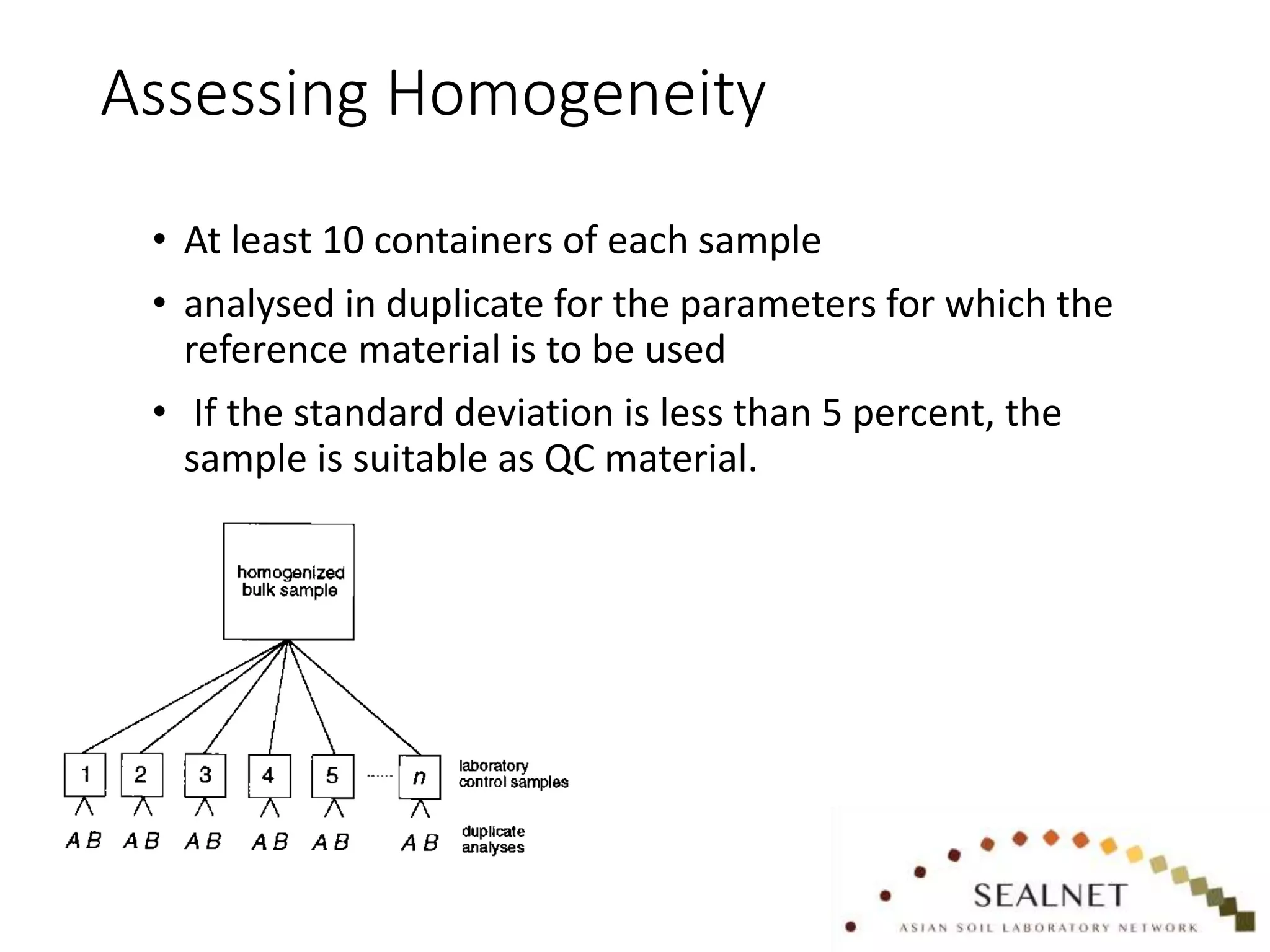
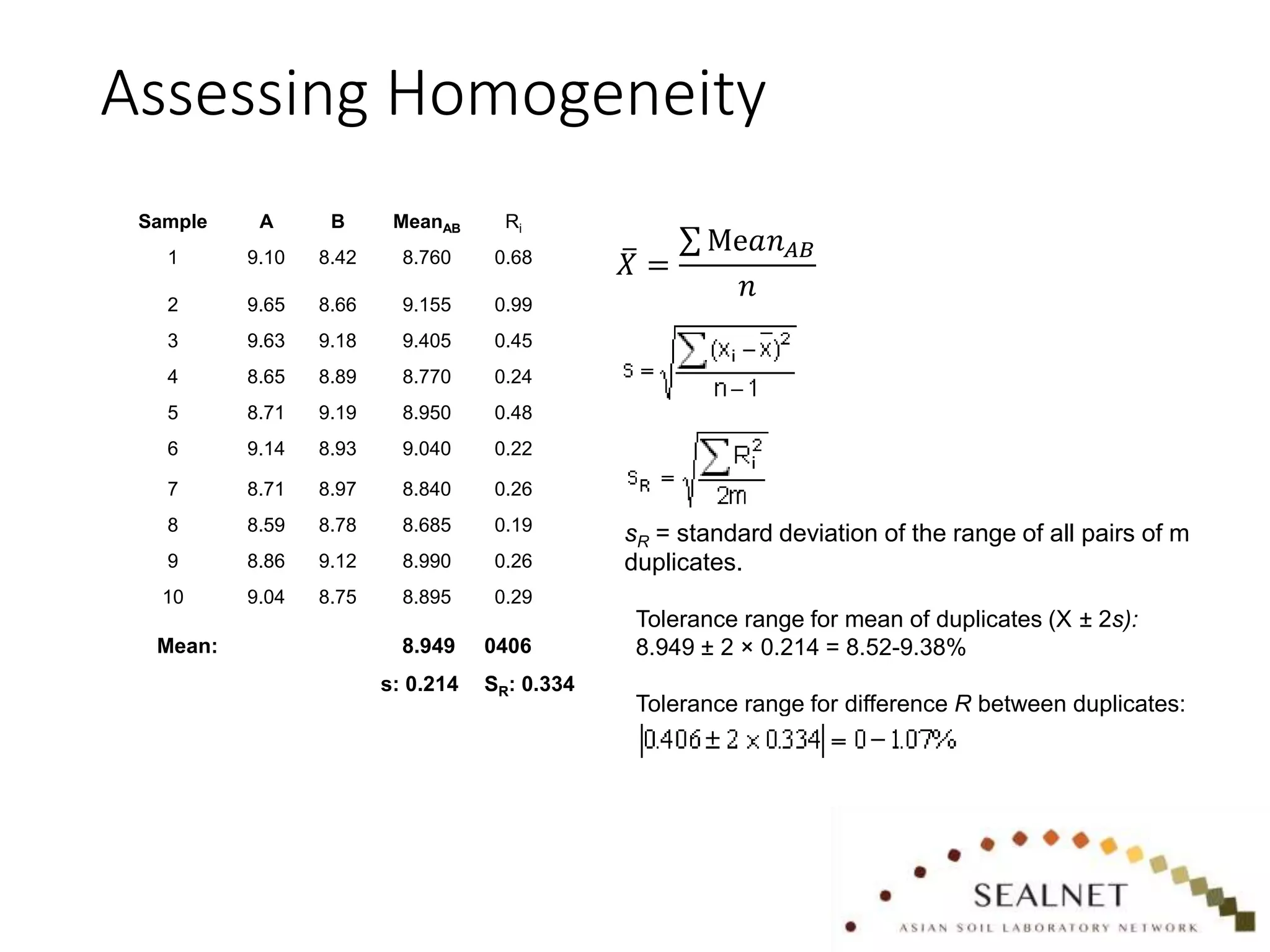
The document outlines procedures for preparing quality control (QC) materials for internal laboratory use, focusing on material selection, preparation, and assessment of homogeneity. It details the steps involved in drying, milling, mixing, and packaging, as well as guidelines for labeling and storage conditions. Key aspects include performing tests to ensure consistency in QC samples and documenting all procedures and conditions to facilitate future reference and compliance with ISO standards.