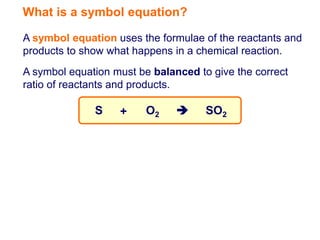
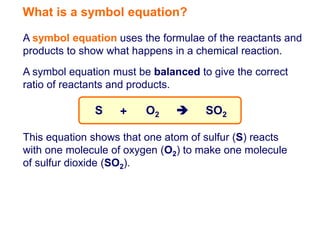
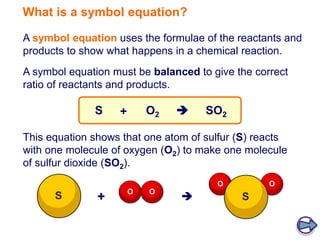
A symbol equation uses chemical formulas to represent reactants and products in a chemical reaction. It must be balanced to show the correct ratio between reactants and products undergoing the reaction. For example, the symbol equation given shows that one atom of sulfur reacting with one molecule of oxygen produces one molecule of sulfur dioxide.