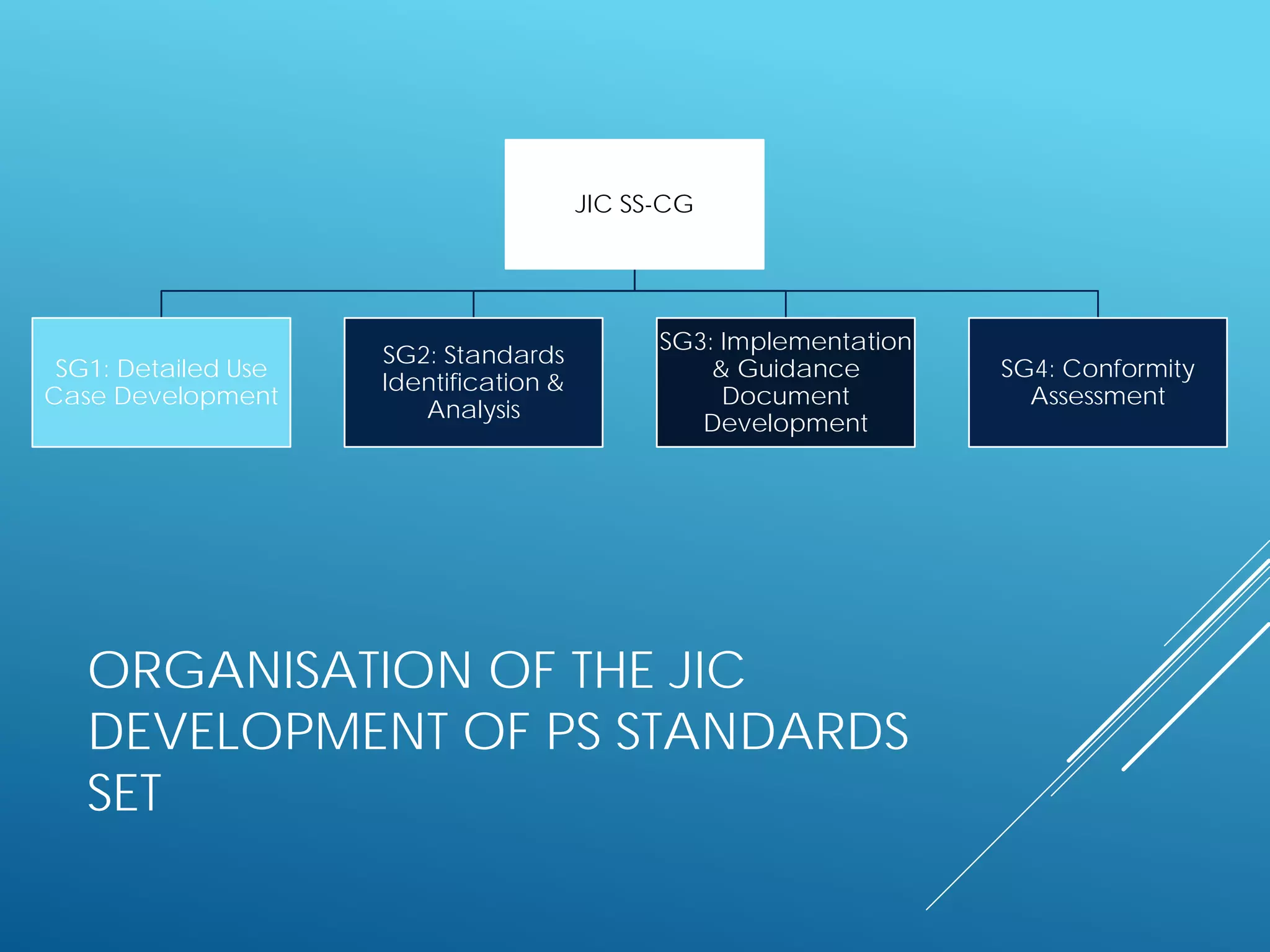
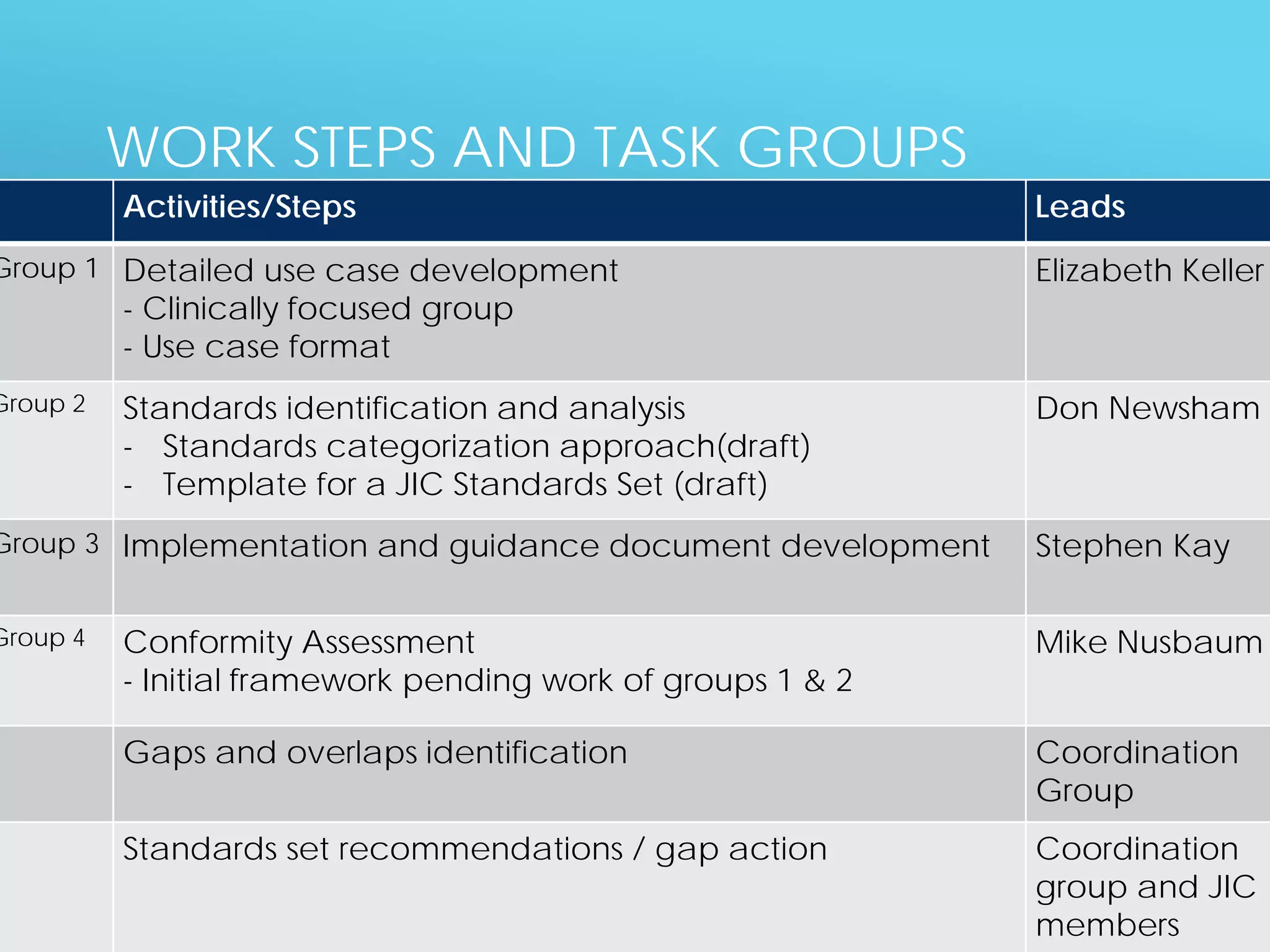
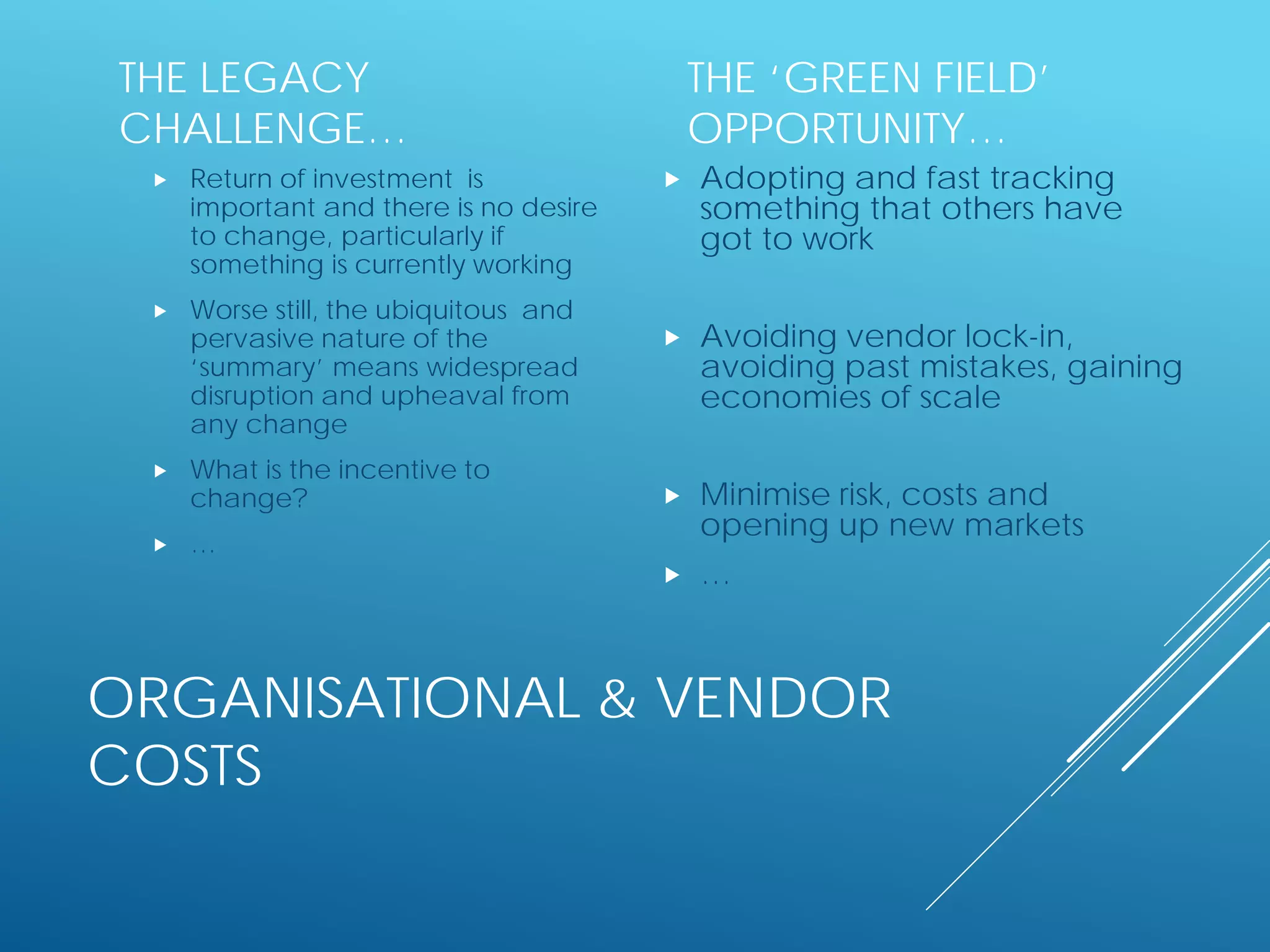
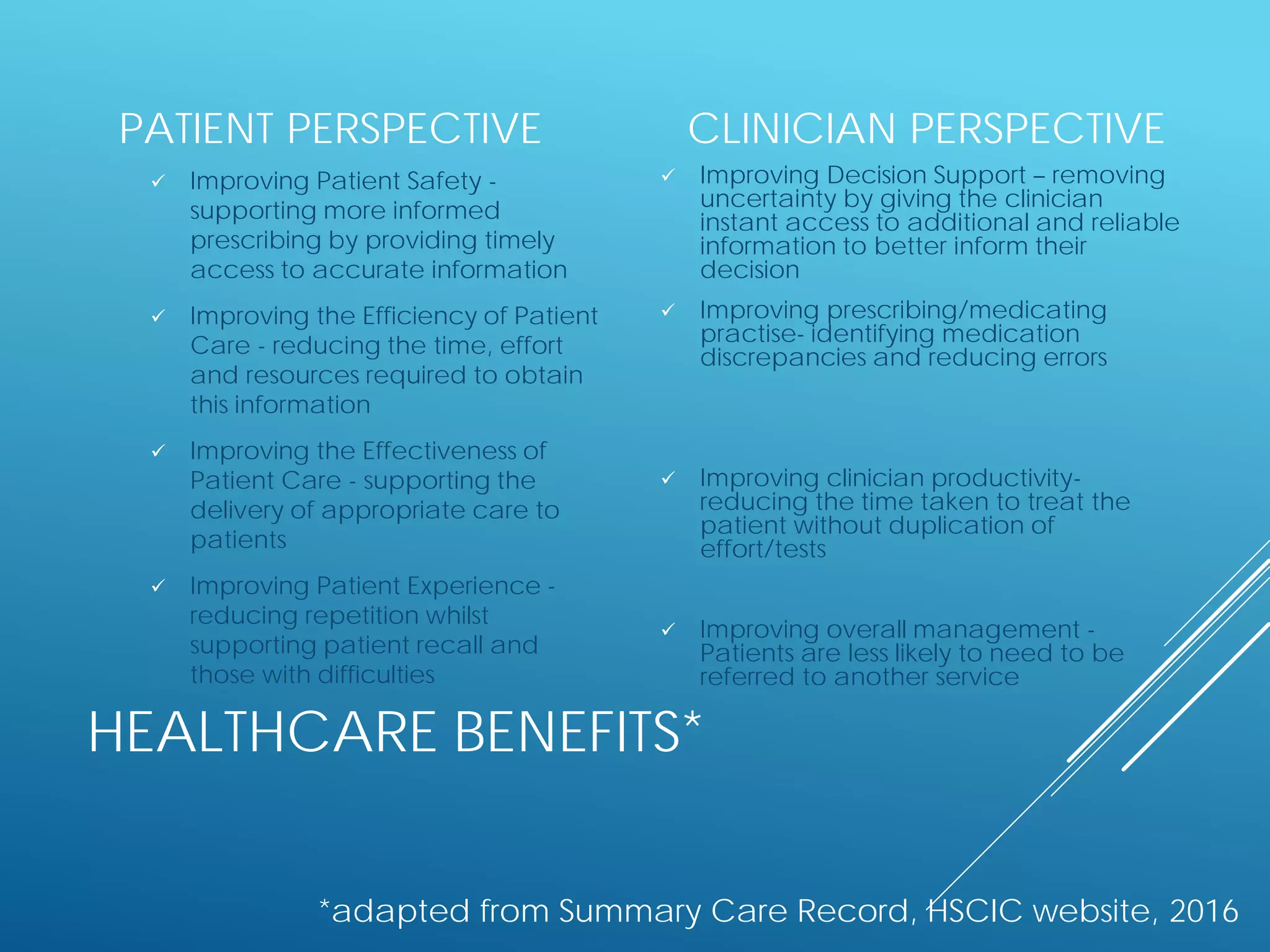
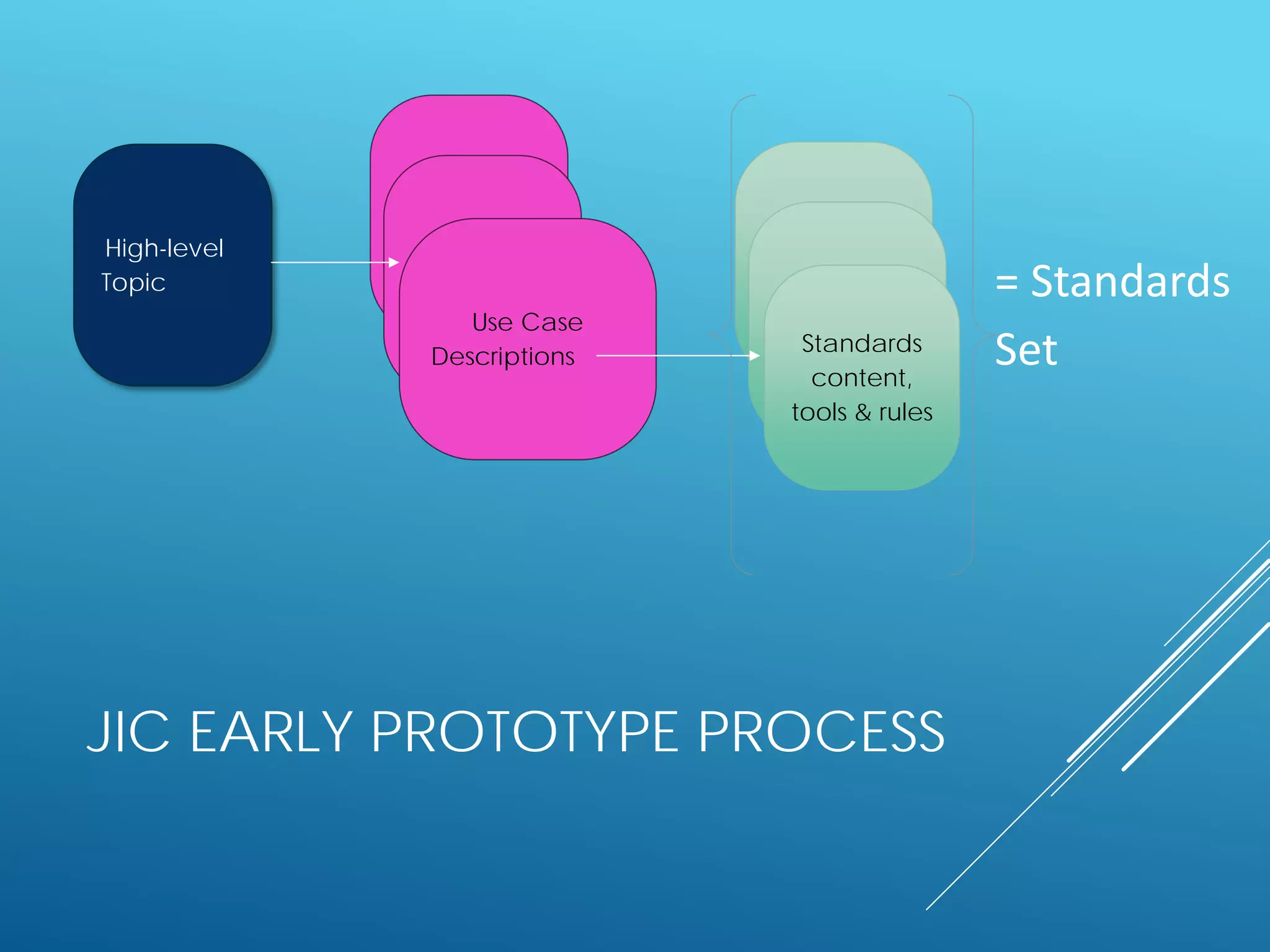
The document discusses international standards for patient summaries. It provides an overview of existing initiatives to standardize patient summaries and the potential benefits these could provide. It describes the Joint Initiative Council's (JIC) work developing an international standard for patient summaries, including establishing working groups to define use cases, identify relevant standards, and provide implementation guidance. The JIC aims to deliver a standardized patient summary within one year by leveraging existing work from other standards development organizations.













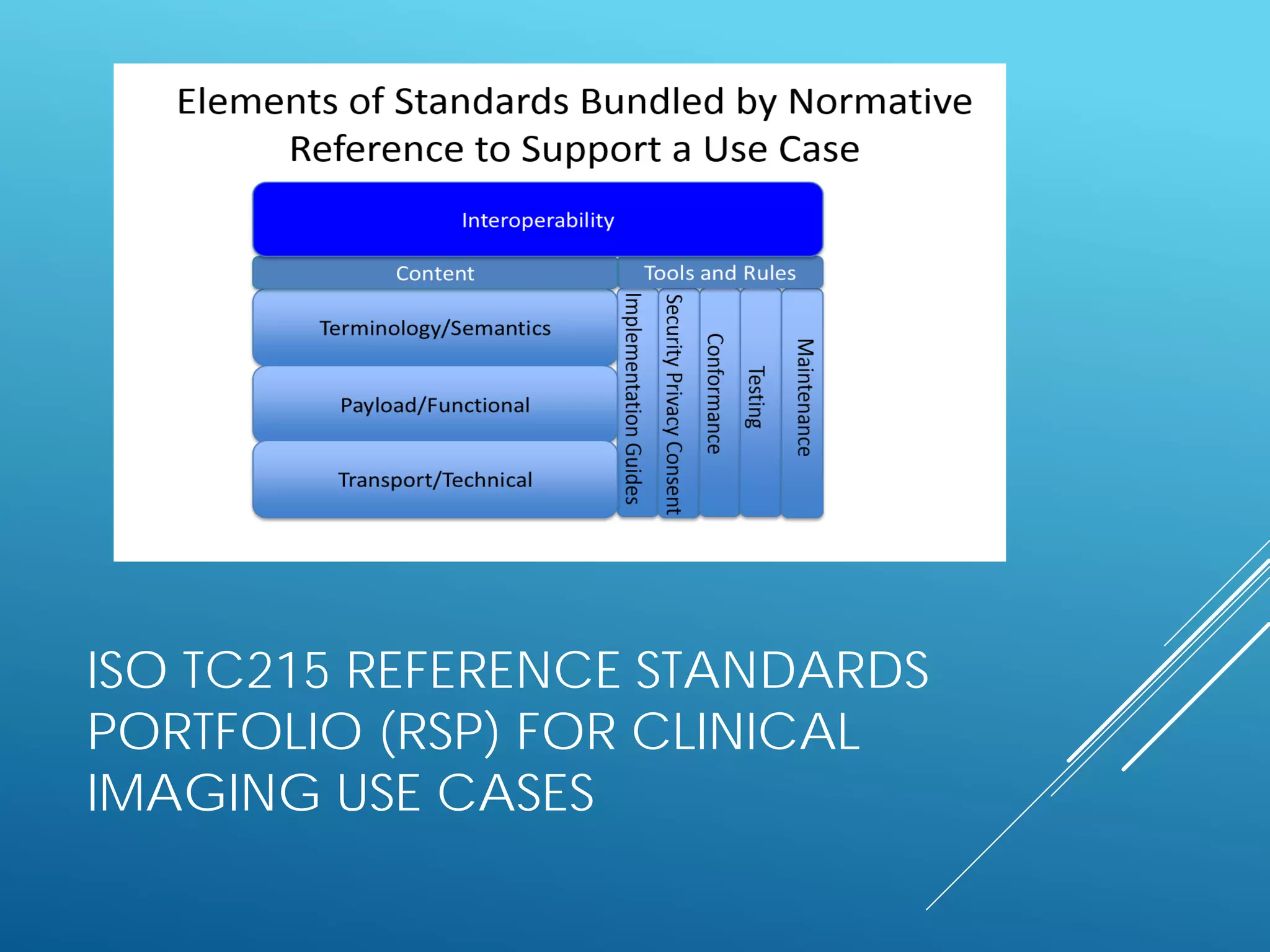



![ADAPTED DEFINITION OF A JIC
STANDARDS SET (SS)
A coherent collection of
[referenced] standards and
standards artefacts that
support a specific use case](https://image.slidesharecdn.com/estandardsconference09patientsummarystandards-160423090615/75/1st-eStandards-conference-next-steps-for-standardization-in-large-scale-eHealth-deployment-Patient-Summaries-22-2048.jpg)