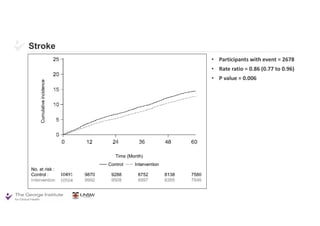
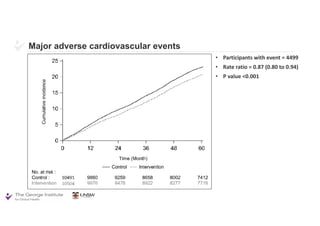
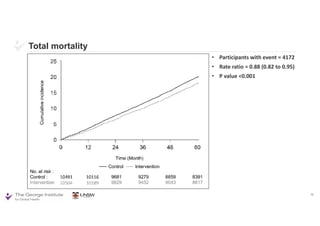
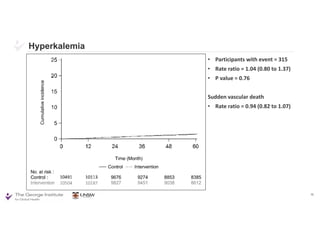
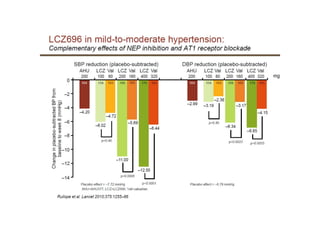
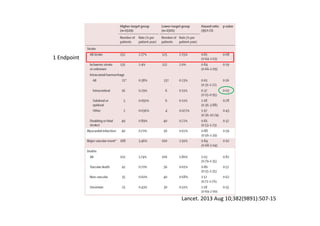
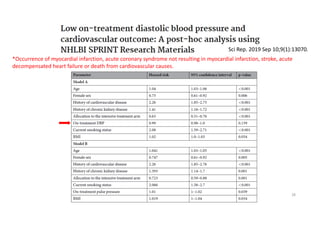
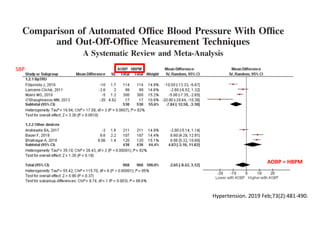
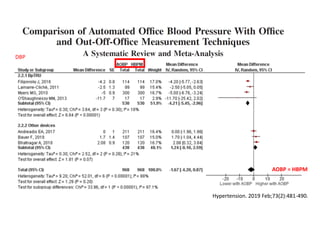
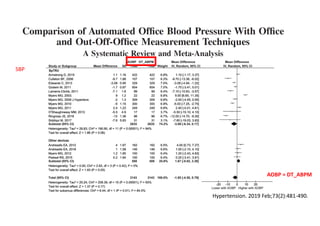
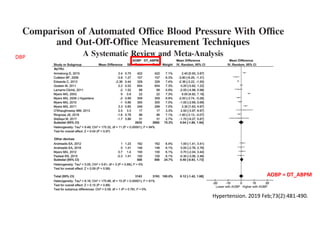
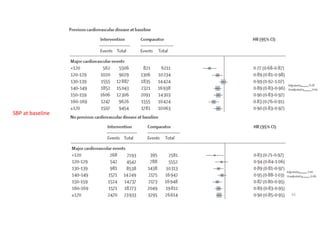
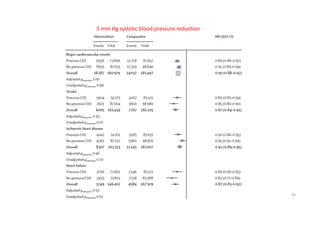
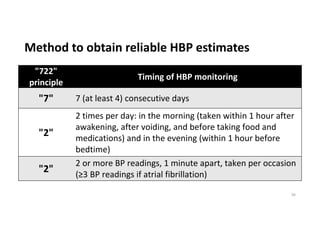
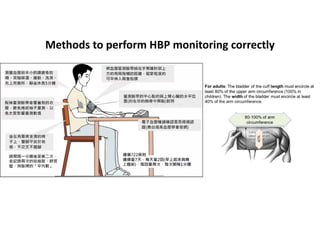
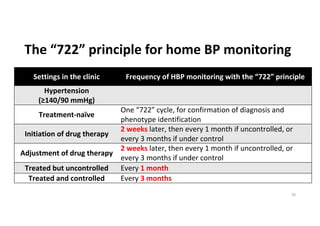
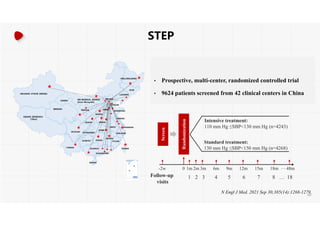
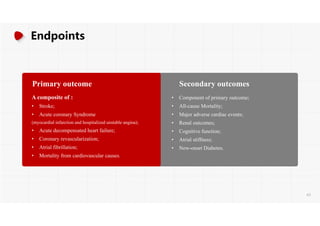
The document summarizes the results of the SPRINT trial, which compared an intensive blood pressure treatment target of less than 120 mmHg systolic to a standard target of less than 140 mmHg. Key findings include:
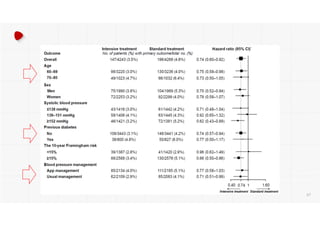
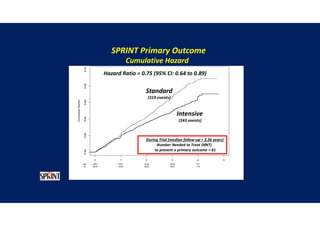
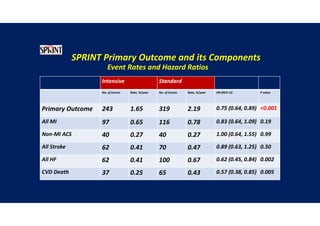
- The intensive treatment group had a significantly lower rate of the primary composite outcome of myocardial infarction, other acute coronary syndromes, stroke, heart failure or death from cardiovascular causes.
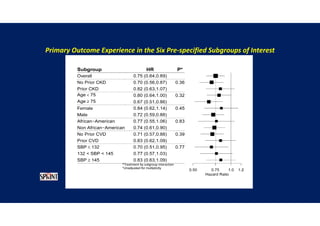
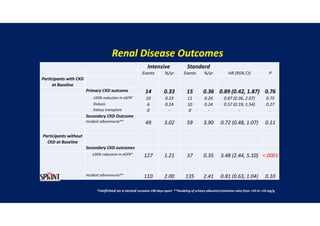
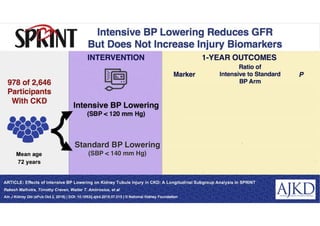
- Benefits of intensive treatment were seen across most pre-specified subgroups including those with and without chronic kidney disease.
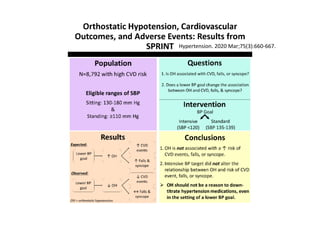
- Intensive treatment led to more frequent adverse events related to low blood pressure like hypotension, but no differences in falls or fractures.
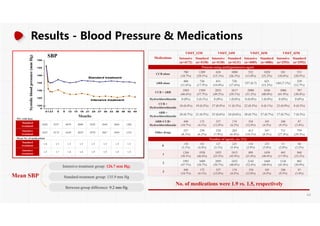
- More medications were required to achieve the lower blood







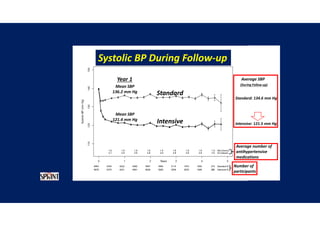




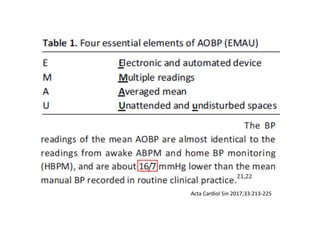







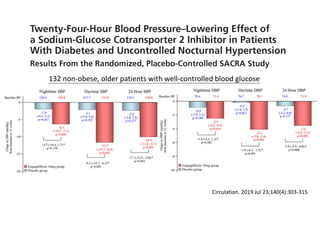
![Time after randomization (Months)
Cumulative
incidence
26%
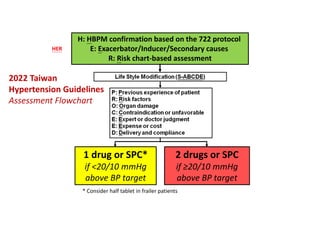
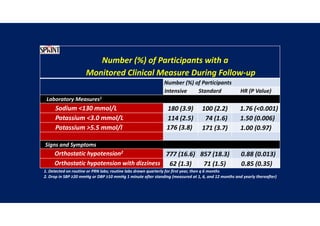
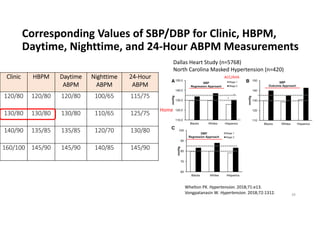
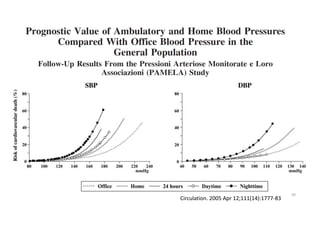
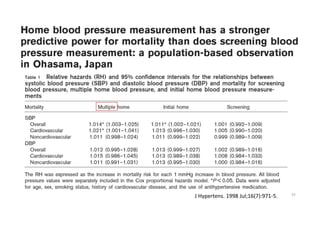
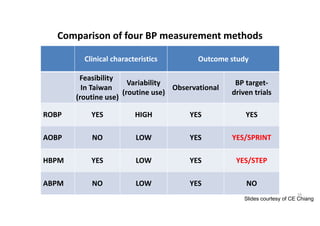
Results- Primary Composite Outcome
Standard
treatment
4268 4147 4070 4000 3938 3849 3664 1200
Intensive
treatment
4243 4174 4109 4039 3970 3867 3694 1234
NO.at risk
The cardiovascular
benefits
65
147 of 4243 patients (3.5% [1.0% per
year]) in the intensive-treatment group;
196 of 4268 patients (4.6% [1.4% per
year]) in the standard-treatment group.
During the median follow-up period of
3.34 years, primary-outcome events
occurred in:](https://image.slidesharecdn.com/1120310-230310085239-30dfc89f/85/1120310-pdf-65-320.jpg)