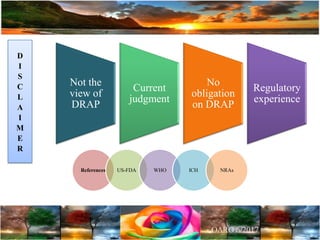
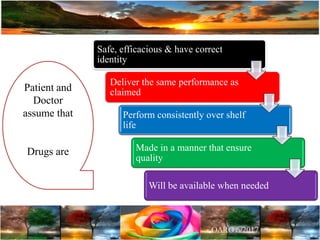
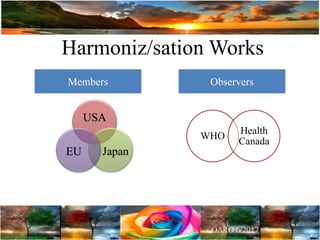
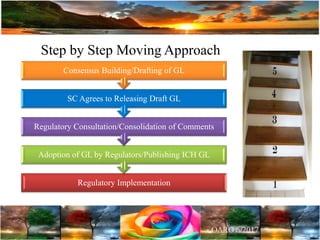
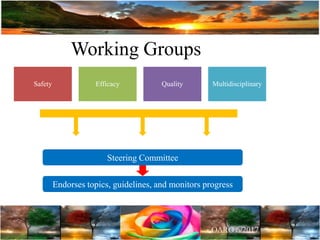
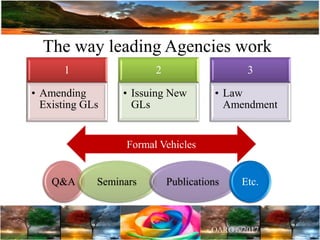
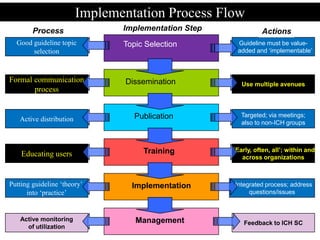
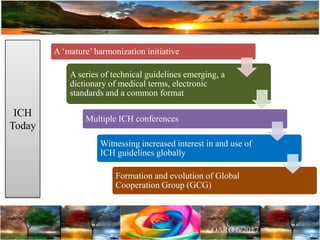
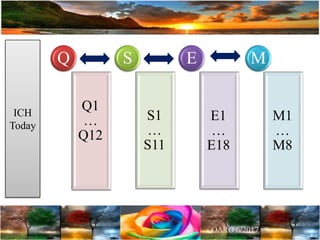
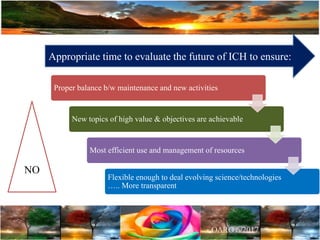
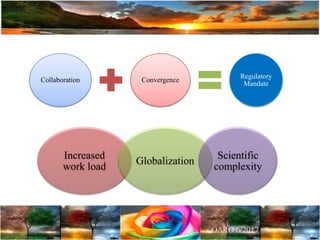
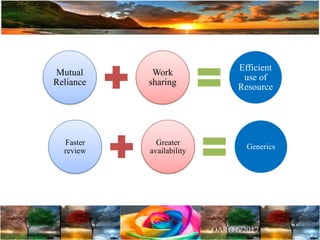
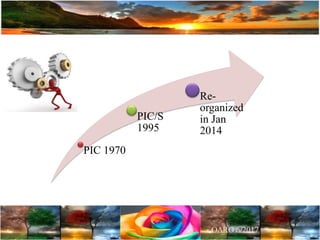
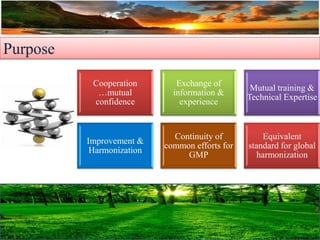
The document discusses the importance of harmonization in pharmaceutical regulatory sciences, emphasizing the need for regulations to ensure the safety, efficacy, and quality of drugs amidst globalization and increasing complexity. It highlights the role of the International Conference on Harmonization (ICH) in streamlining drug registration processes between the US, EU, and Japan while identifying the value of collaboration and transparency in regulatory practices. The document also concludes with a vision for continued global cooperation and the evolution of guidelines to cater to emerging scientific advancements.