The document provides an introduction to energy management concepts including:
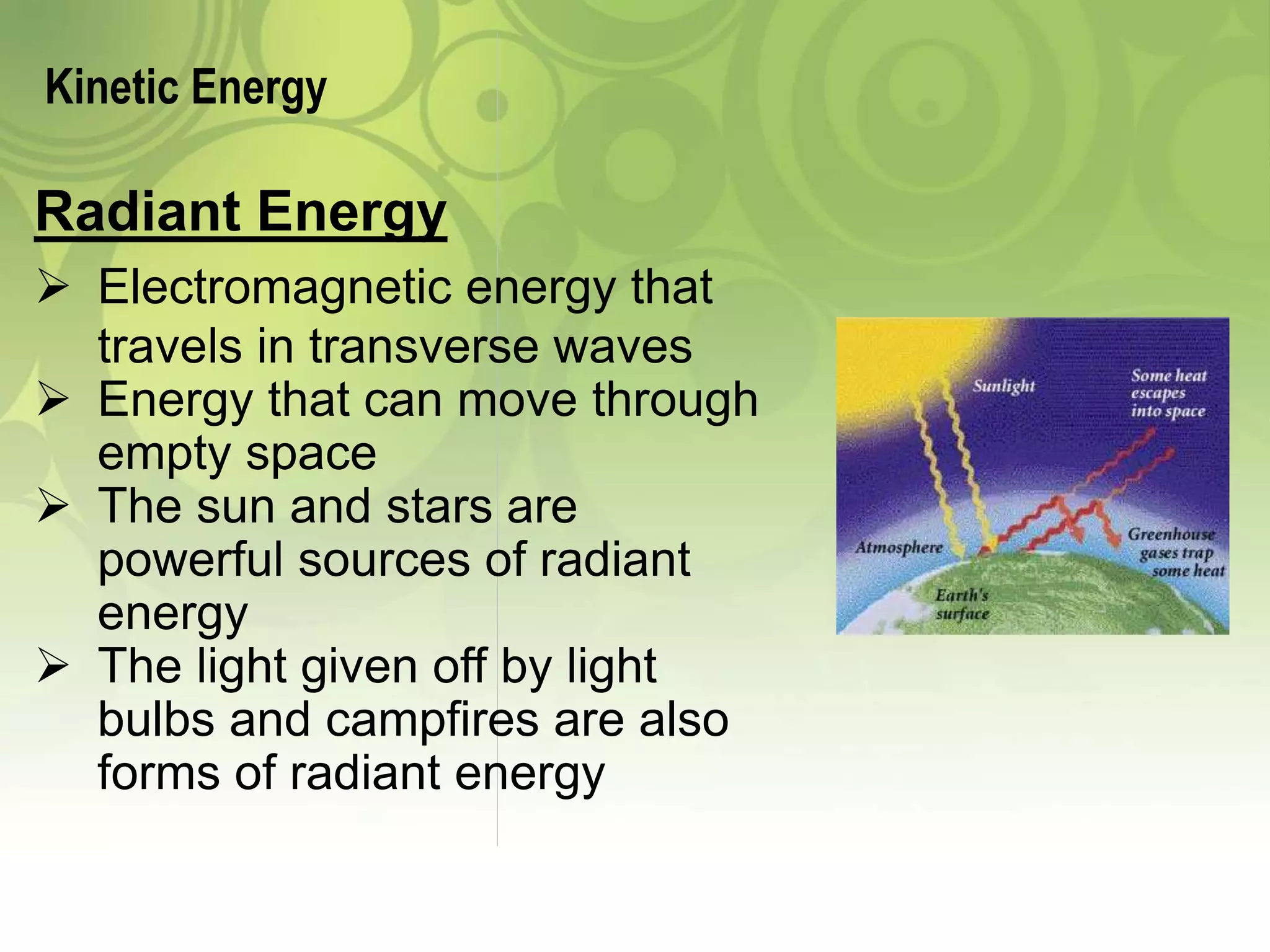
- Different forms of energy including potential (chemical, nuclear, gravitational, elastic) and kinetic (mechanical, electrical, thermal, radiant, sound) energy.
- Energy transformations are never 100% efficient and energy is conserved by changing forms but not being created or destroyed.
- Examples of energy transformations include burning fuel, solar panels generating electricity, photosynthesis, and cars gaining thermal energy from motion.