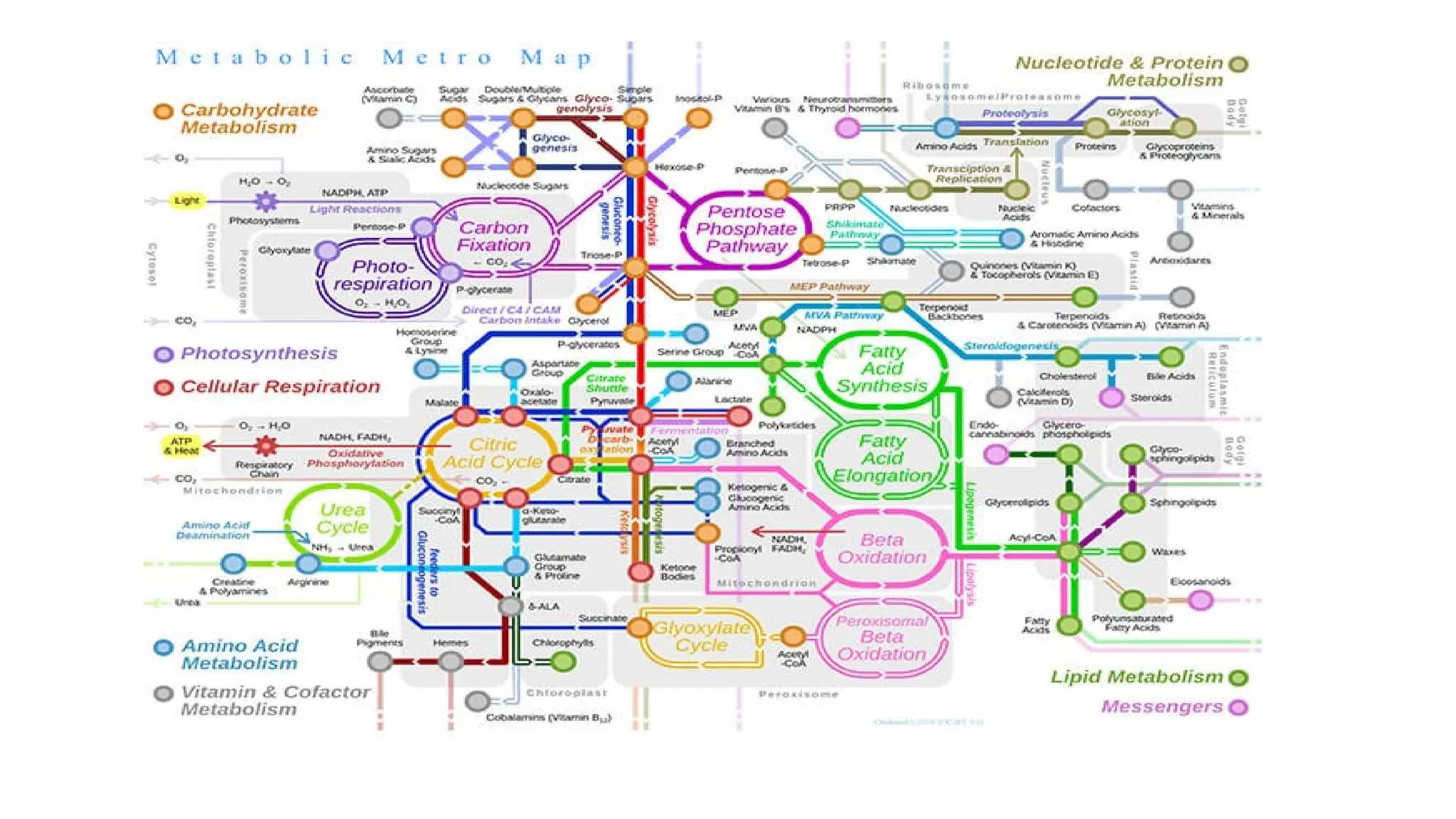
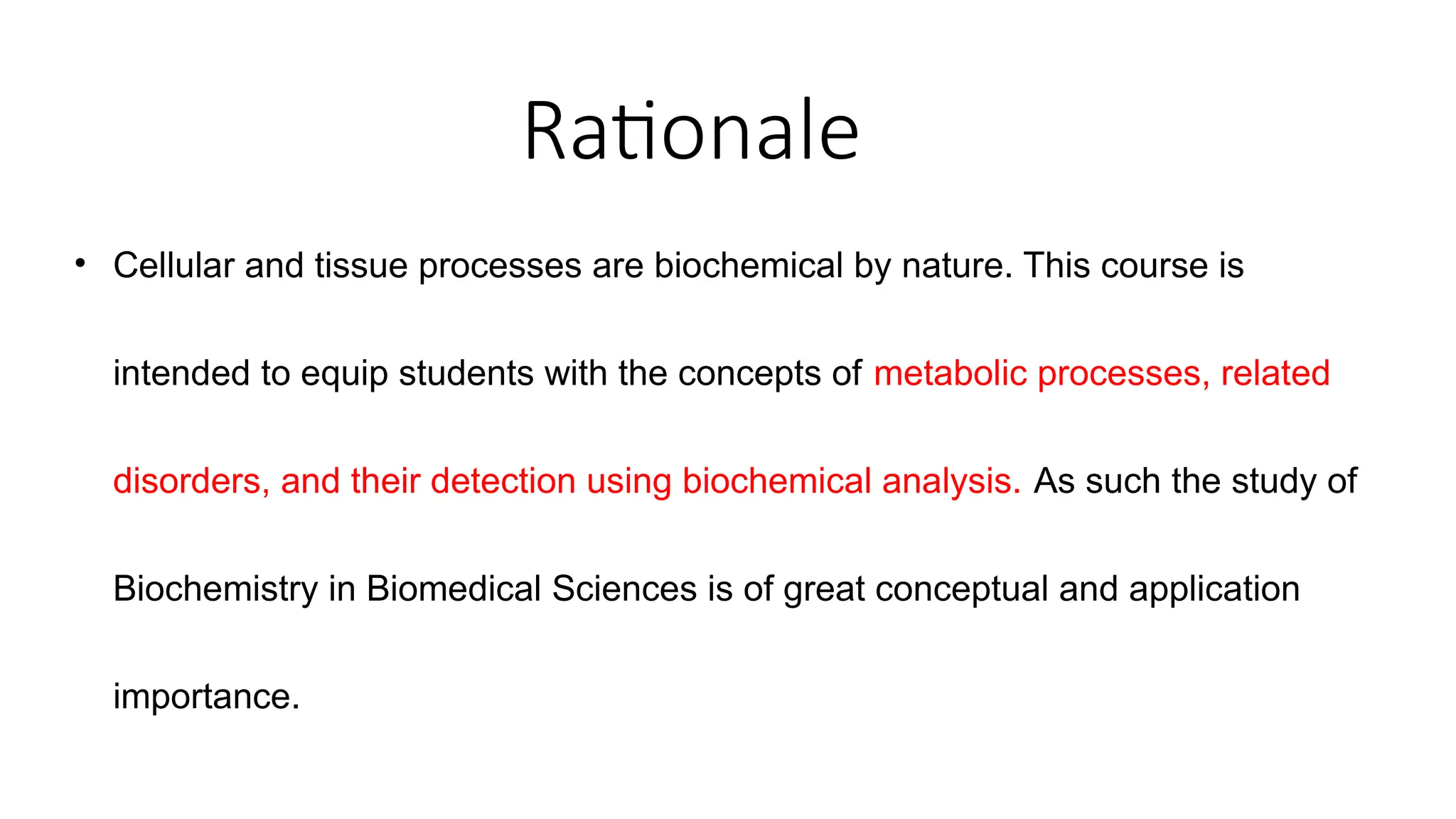
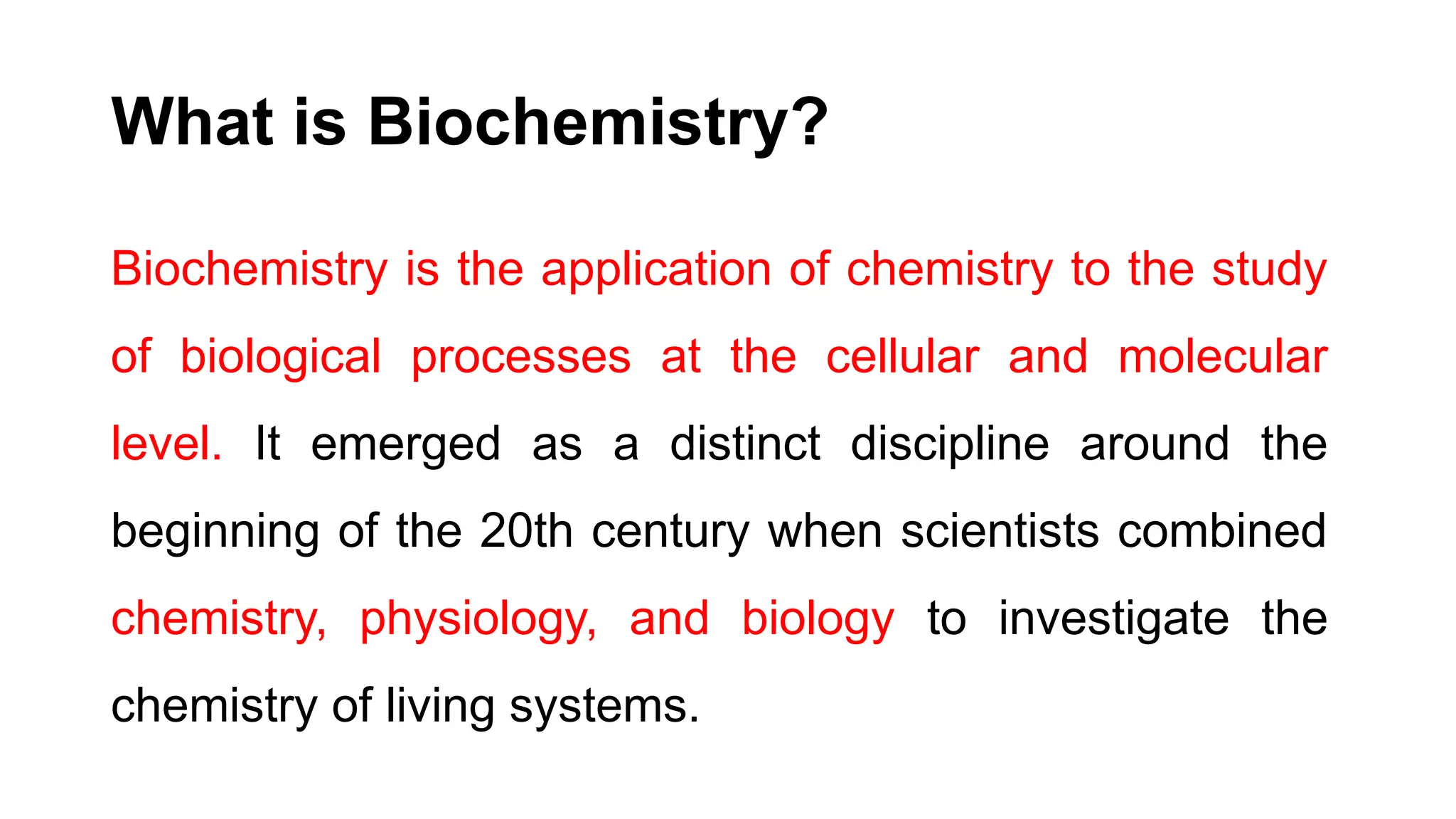
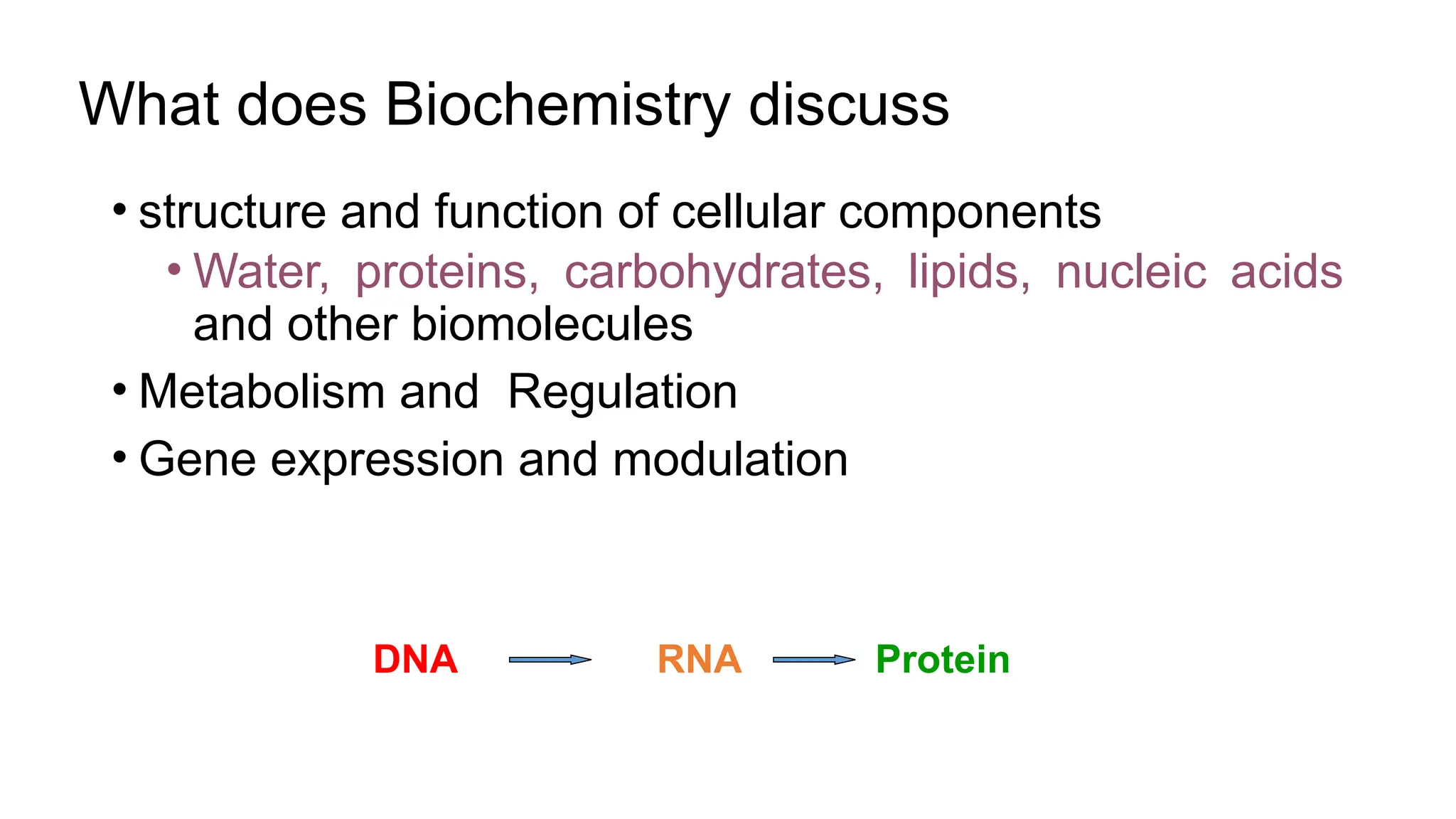
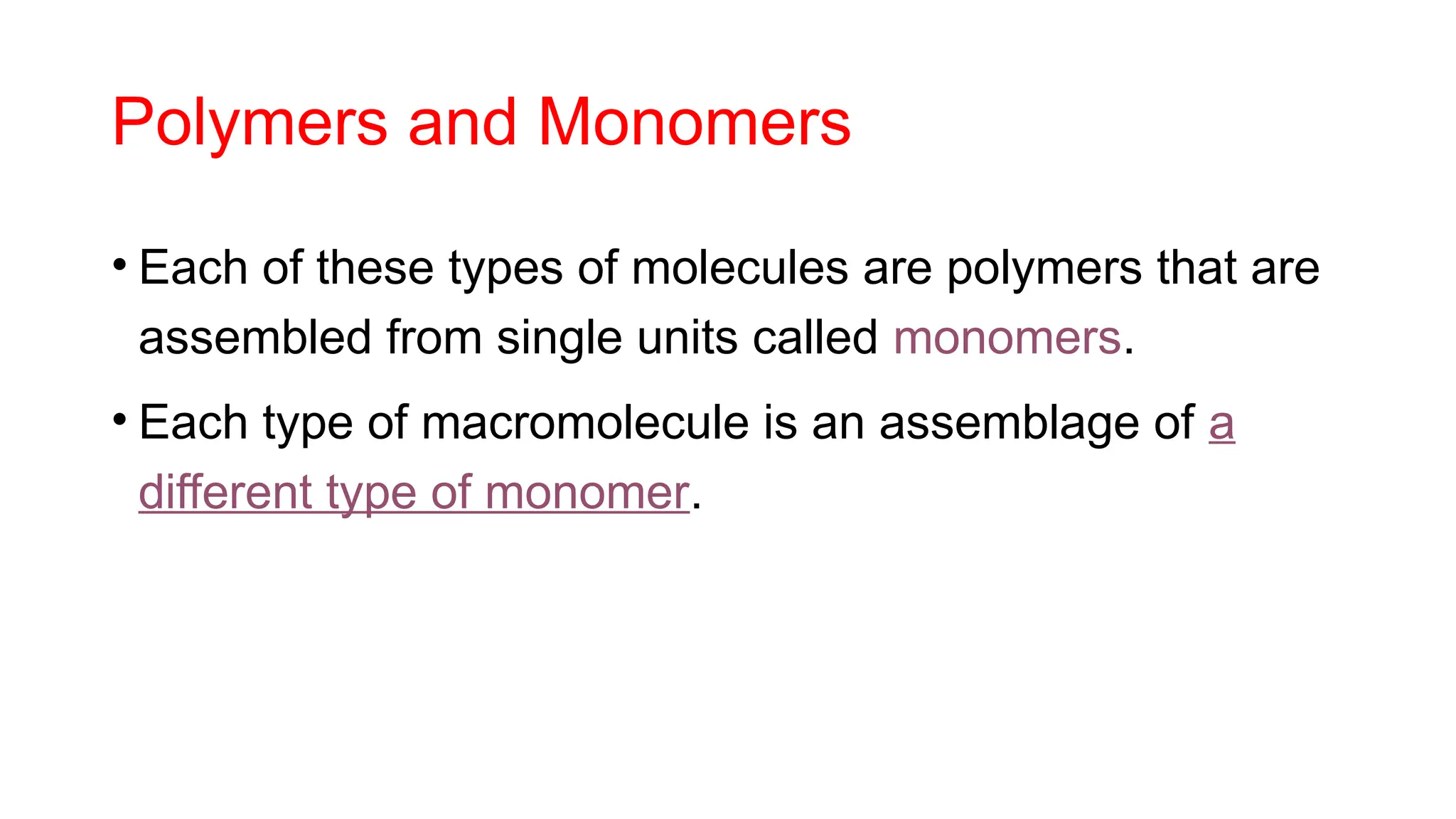
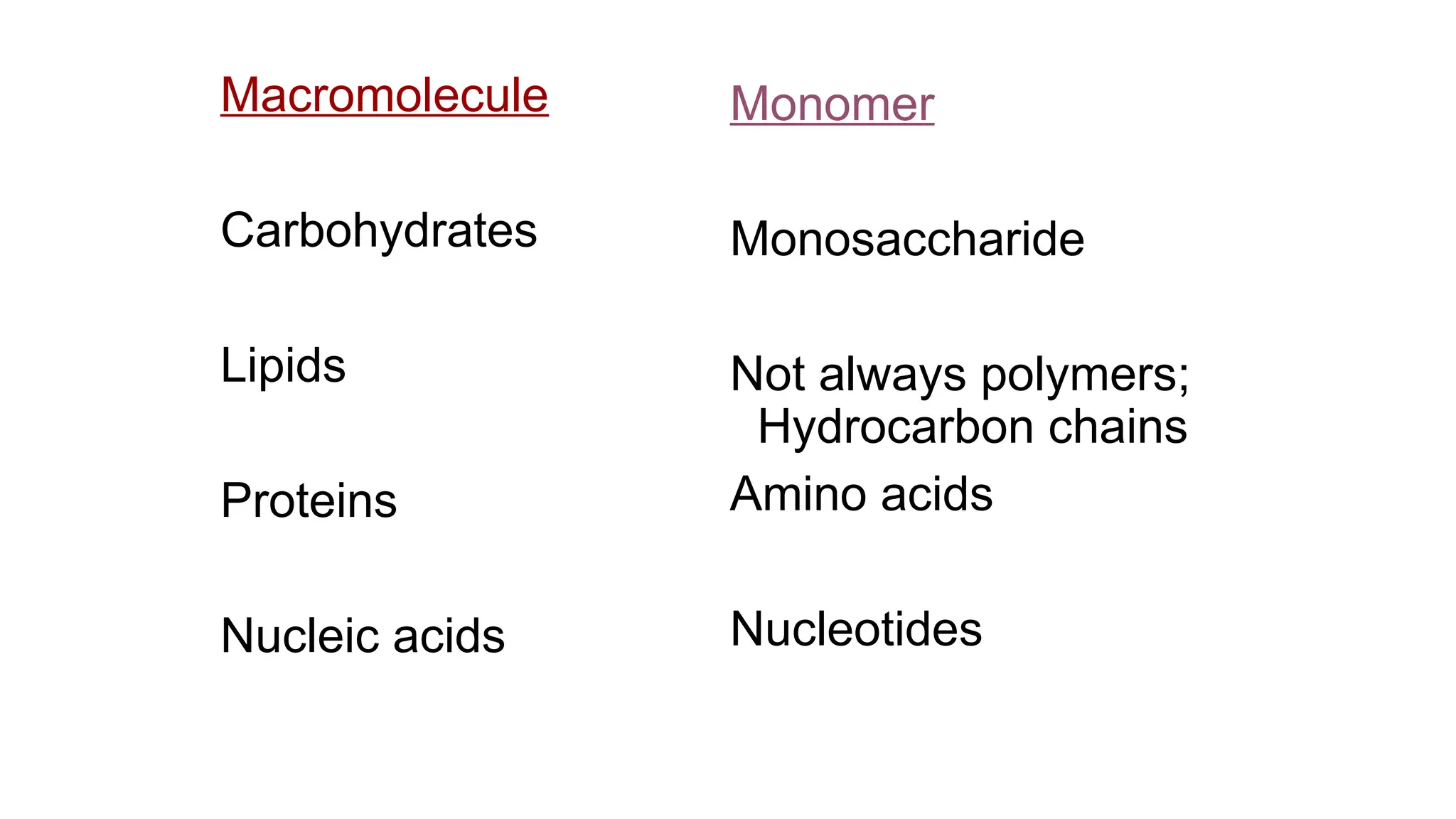
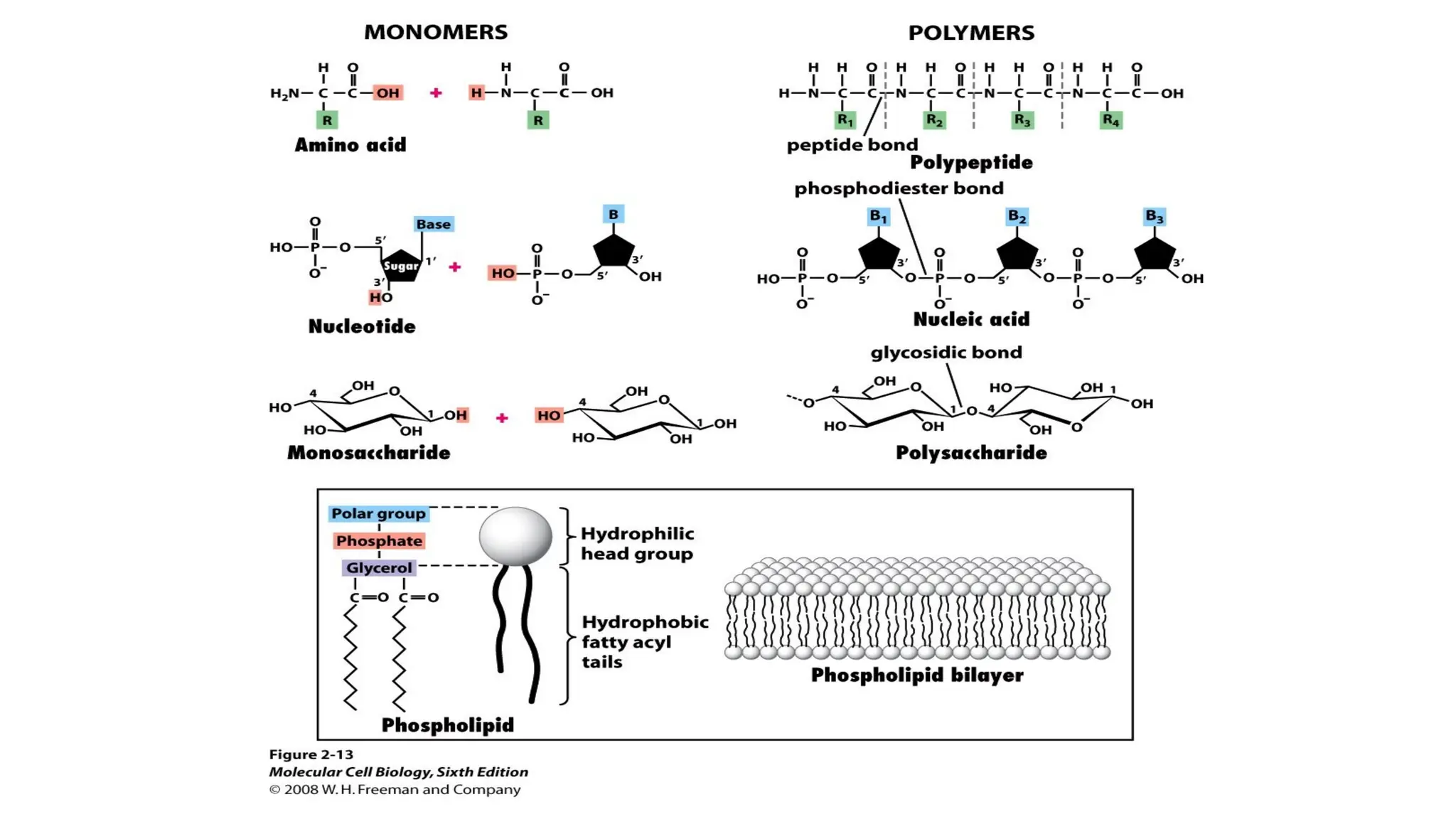
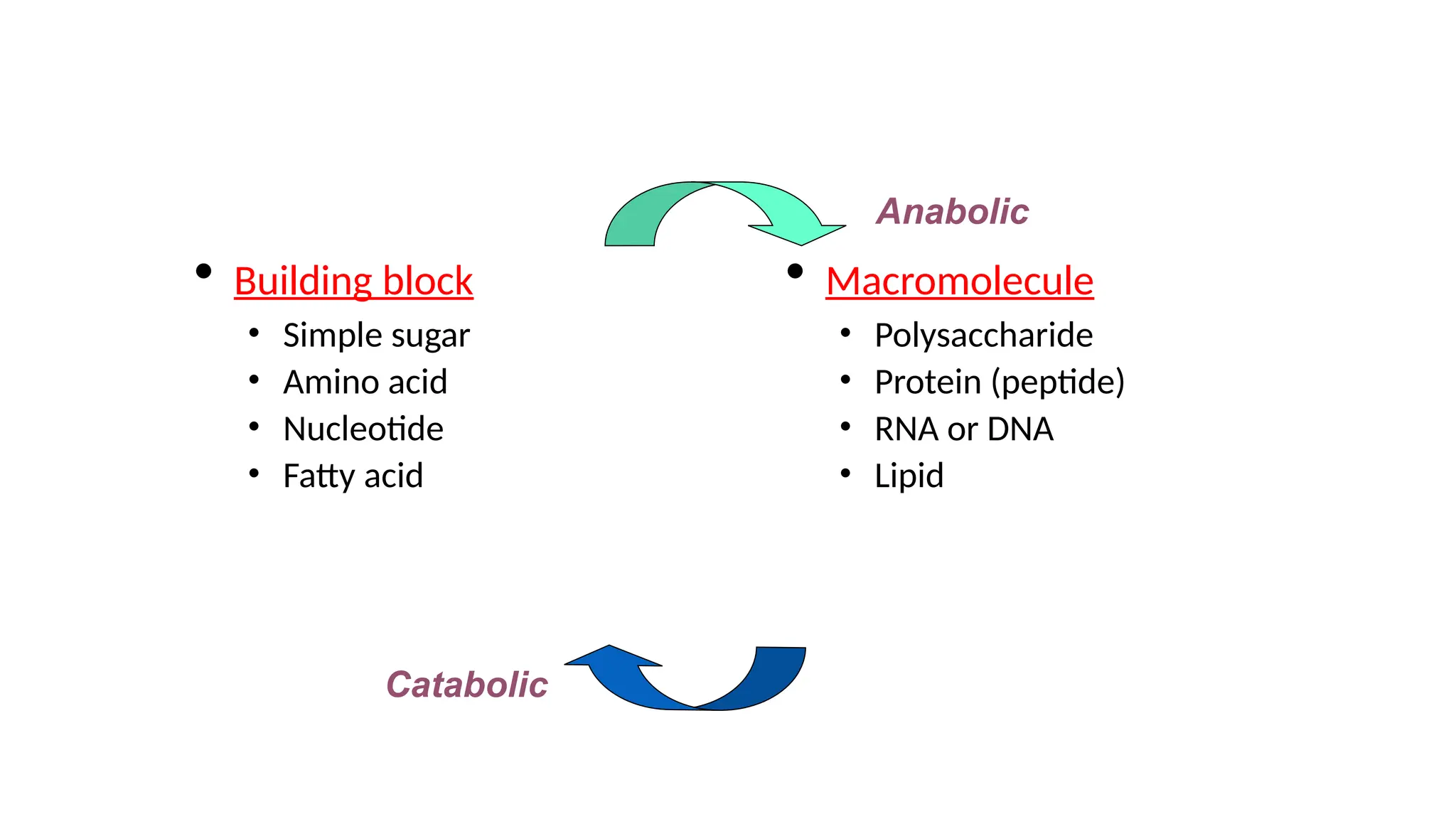
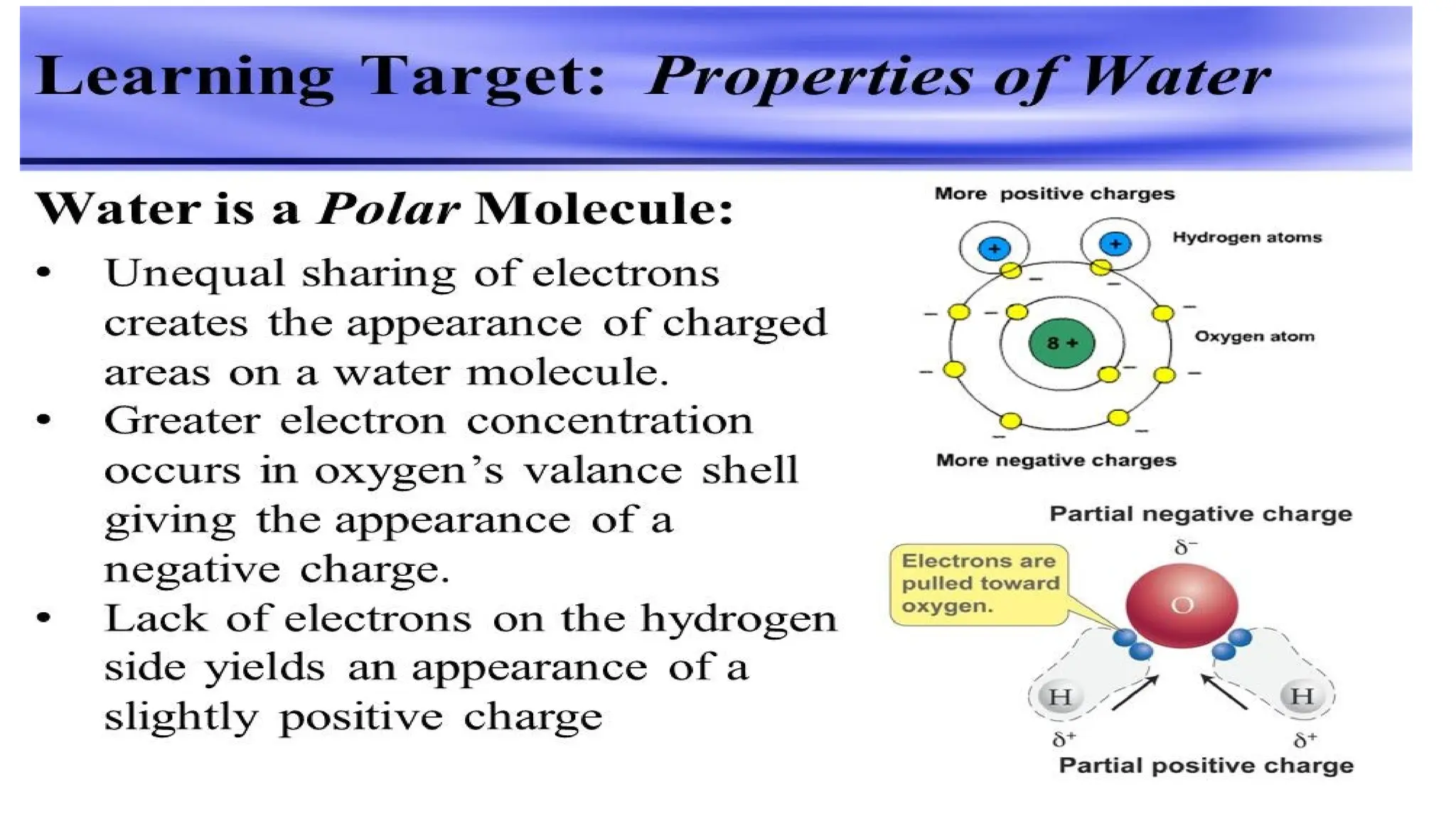
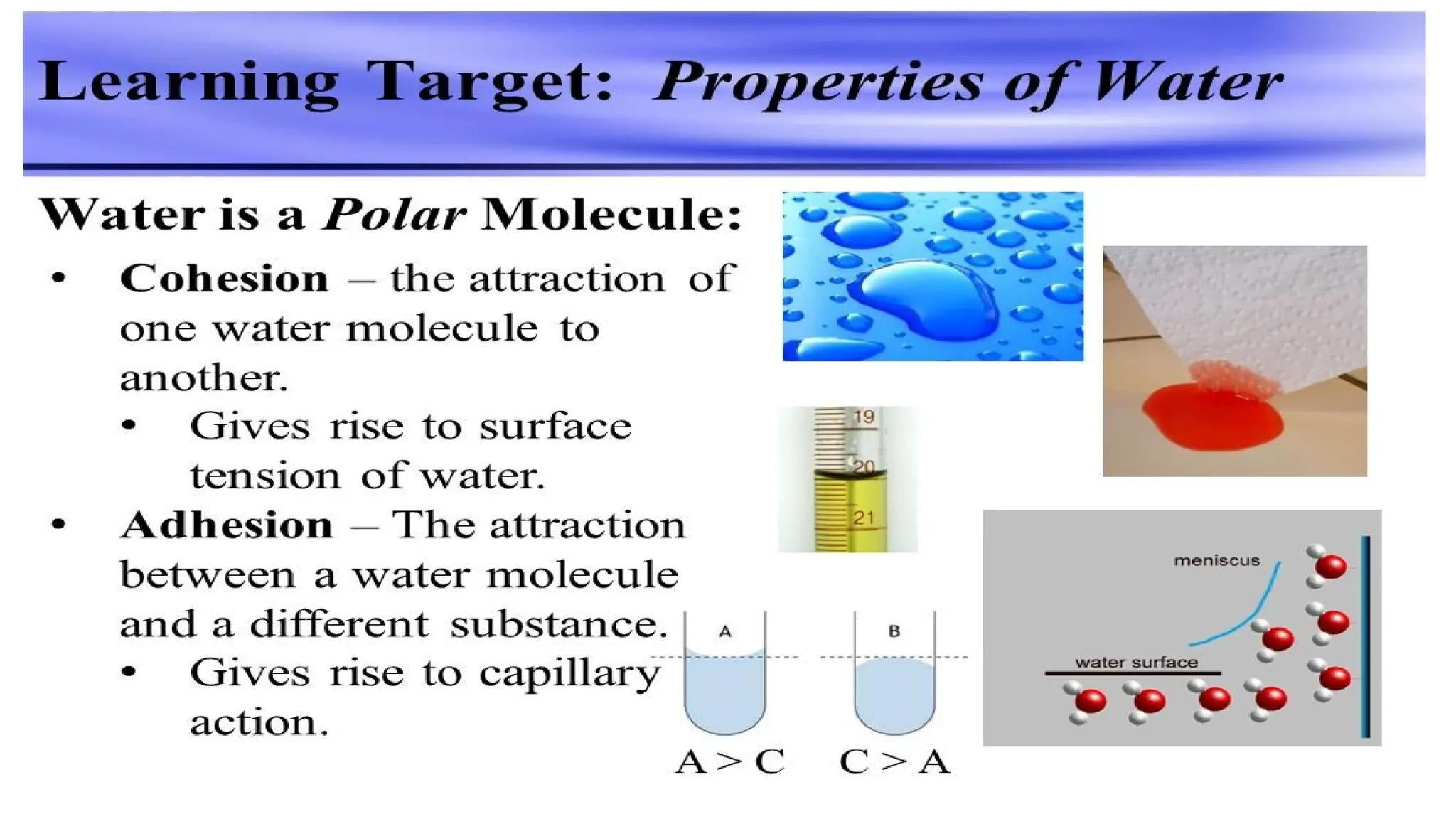
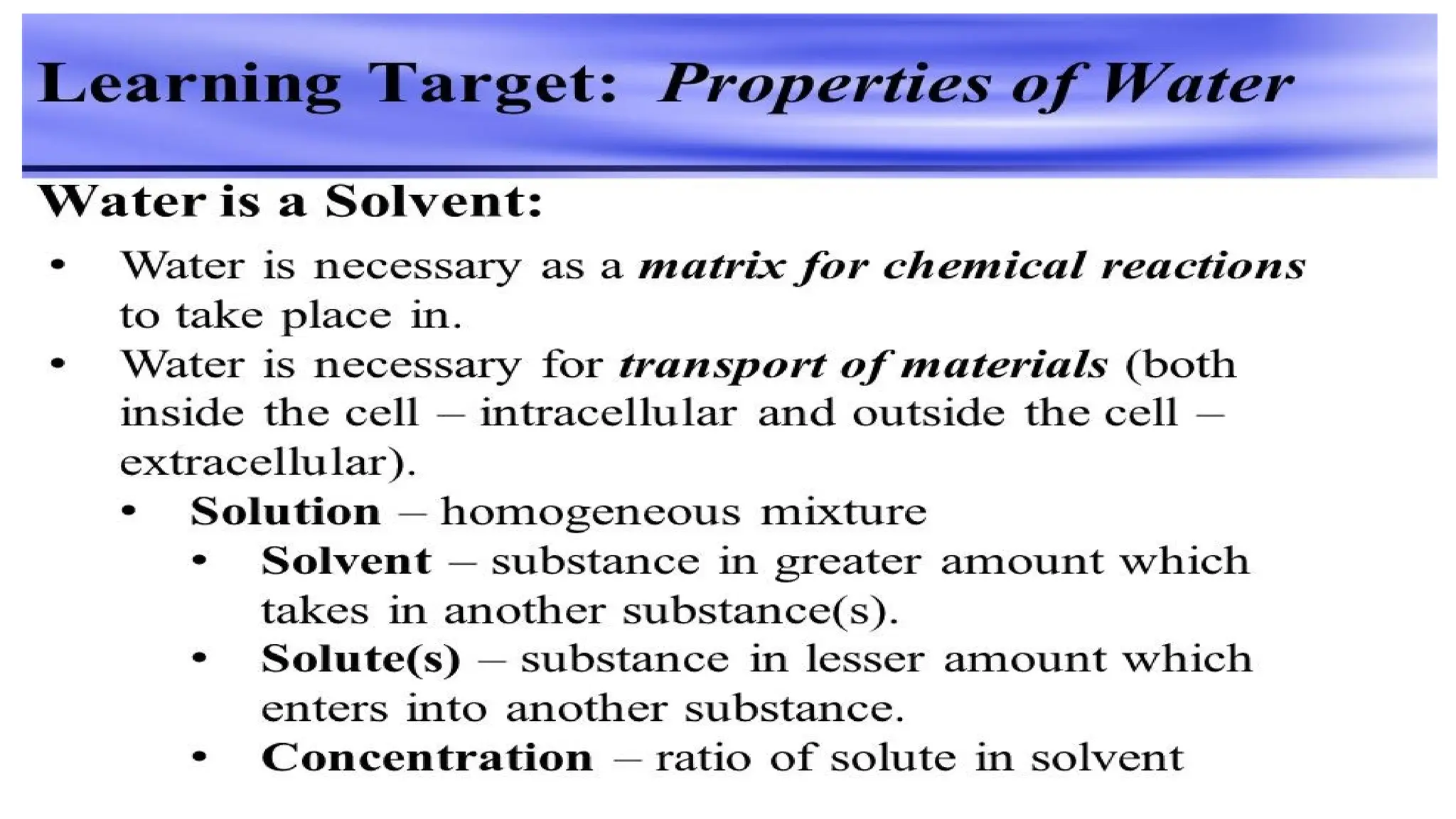
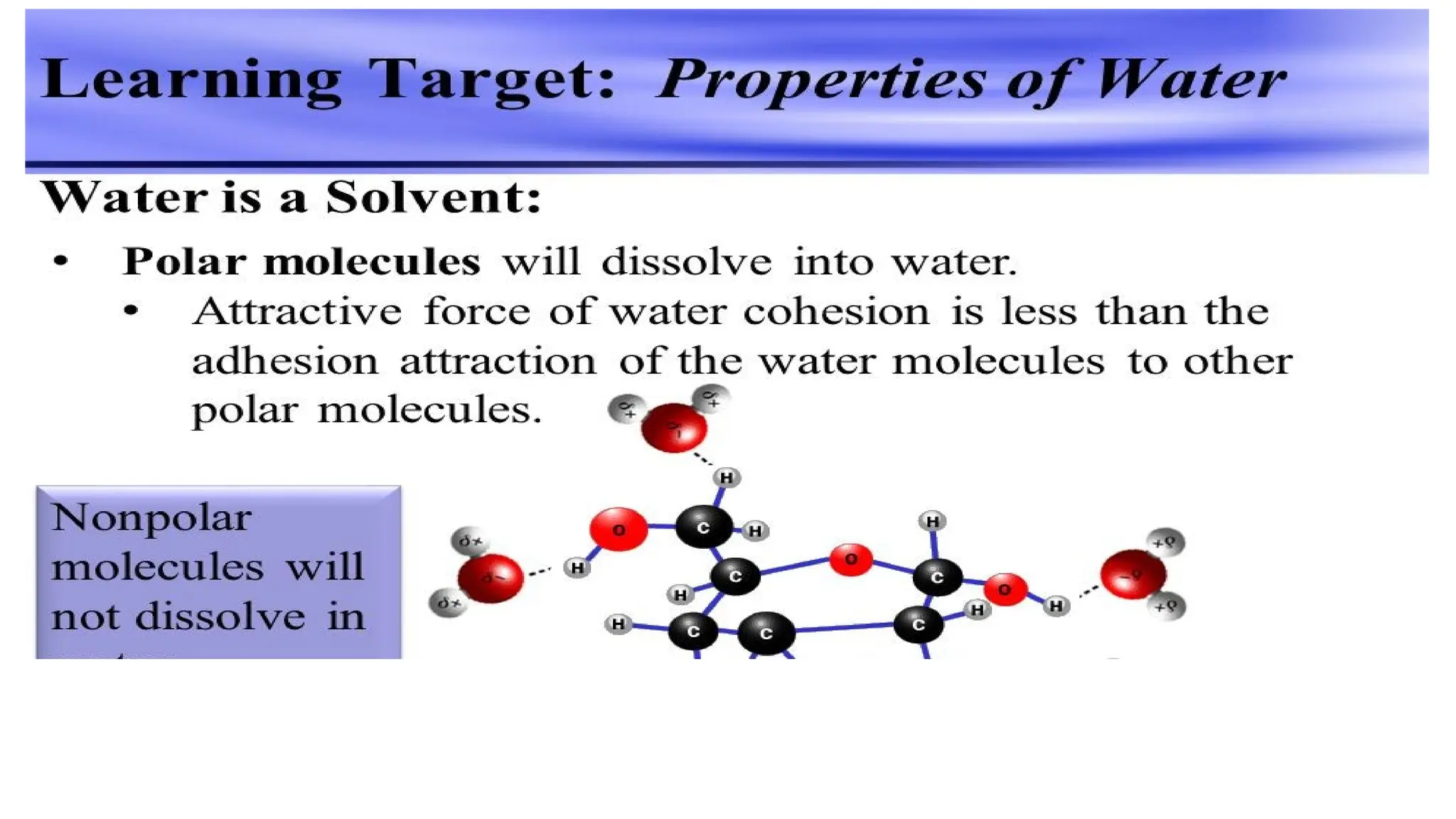
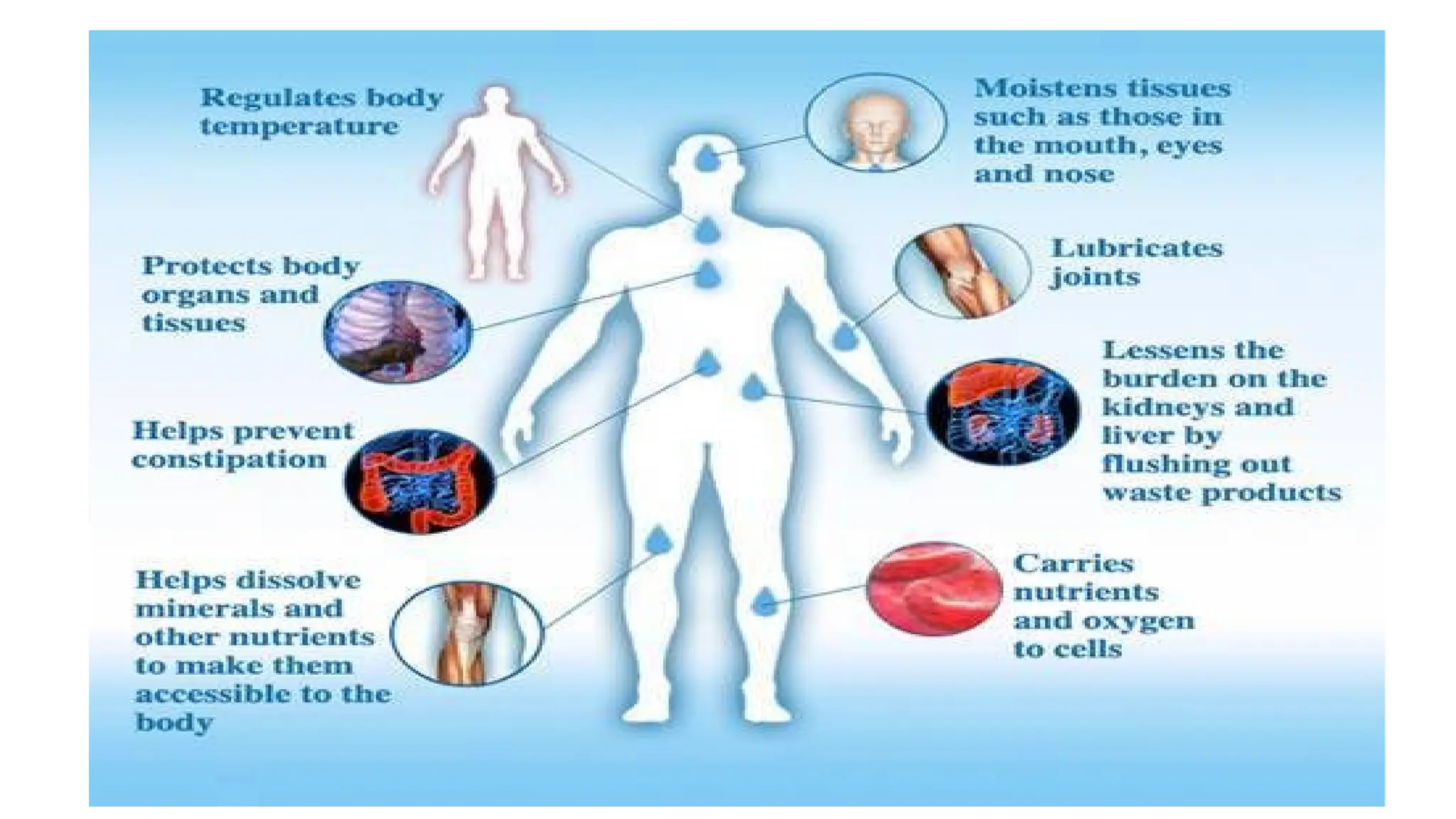
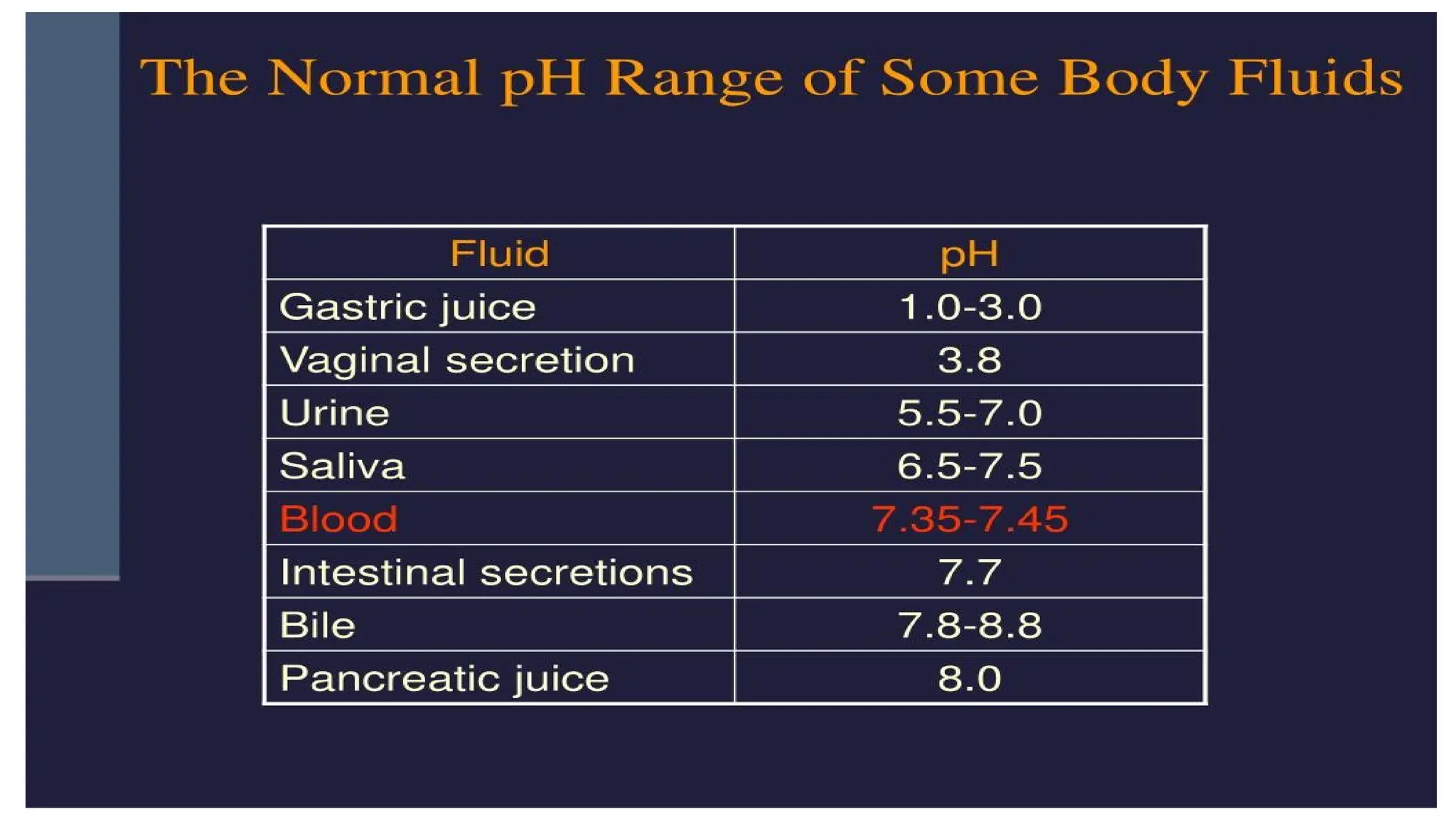
The document outlines an introductory course on general and metabolic biochemistry, focusing on biochemical processes in cellular and tissue functions. It aims to equip students with knowledge about metabolic processes, enzyme functions, biomolecule classifications, and energy metabolism. Key objectives include understanding the roles of water, biomolecules, and regulatory mechanisms in biochemical systems.