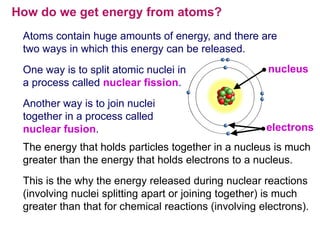
Albert Einstein first came up with the idea of getting energy from atoms. Scientists found that large amounts of energy could be produced through the process of nuclear fission, which involves splitting atoms of uranium. There are two ways to release the huge amounts of energy contained within atoms: nuclear fission, which is splitting atomic nuclei, and nuclear fusion, which is joining atomic nuclei together. Einstein connected energy and matter through his famous equation E=mc2, which showed that a small amount of matter can release a huge amount of energy.