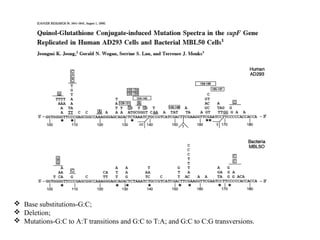
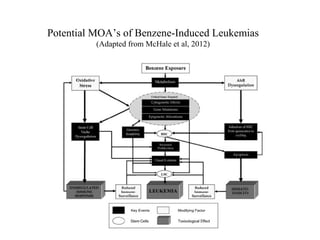
The document discusses benzene's role in leukemogenesis, focusing on its metabolic processes and mechanisms that lead to hematotoxicity and leukemia. It explores the evidence for a mode of action in animals and its relevance to humans, while outlining the key metabolic pathways and reactive metabolites associated with benzene. The conclusion emphasizes the potential risks of benzene exposure and the need for further research to understand its effects on human health.




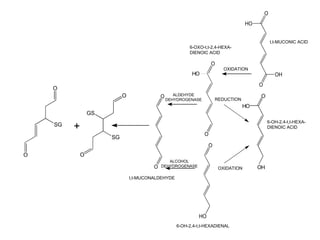
![OH
S-PHENYL-GSH 6-OH-tert,tert-HEXA-
DIENOIC ACID
SG CHO CHO COOH
tert,tert-MUCONALDEHYDE
OH +
tert,tert-MUCONIC
H ACID
1-(GSyl)-CYCLOHEXA-
3,5-DIEN-2-OL H OHC HOOC HOOC
GSH
SG
RING OPENING H
EPOXIDE
OH
CYP2E1 HYDROLASE
O O
H
NADPH OH
BENZENE NADP
BENZENE BENZENE
OXIDE OXEPIN DIHYDRODIOL
O OH OH OH DEHYDROGENASE
OH
[O] [O] [O]
1,4-BENZOQUINONE
OH
PHENOL CATECHOL
O
HYDROQUINONE
GSH
OH
GLUCURONIDE & SULFATE
CONJUGATES
SG +
OH
GS-HQ
OH
OH 1,2,4-BENZENETRIOL
[O] OH
O OH O OH O
SG SG SG SG SG
GSH [O] GSH [O]
GS-1,4-BQ
GS GS GS SG
GS SG
O OH O
OH O
2,5-GS-1,4-HQ 2,5-GS-1,4-BQ 2,3,5-GS-1,4-HQ 2,3,5-GS-1,4-BQ
(TGHQ)](https://image.slidesharecdn.com/09terrencemonks-121210151658-phpapp02/85/09-terrence-monks-6-320.jpg)


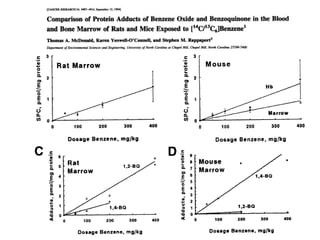
![Benzene Metabolites Identified in Bone Marrow
Benzene (50 ppm for 6hrs):
• Phenol and HQ glucuronides (B63CF1 mice, not rats
[urinary metabolites]).
• Phenol/catechol sulfate
• trans,trans-muconic acid.
(Sabourin et al., 1988).
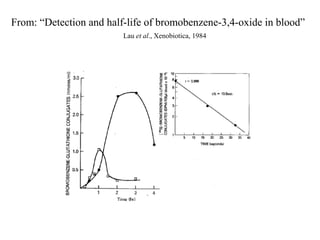
Benzene oxide-derived protein adducts in bone marrow:
[14C13C6]Benzene (50-400 mg/kg)
• 1,4-Benzoquinone - (Mice > Rat)
• 1,2-Benzoquinone - (Rat > Mouse)
• Benzene oxide – identified as phenyl cysteine.
(McDonald et al., 1994)](https://image.slidesharecdn.com/09terrencemonks-121210151658-phpapp02/85/09-terrence-monks-13-320.jpg)

![Superoxide Generation by HQ and GS-HQ Conjugates
70 70
S u p e ro x id e A n io n G e n e ra tio n
r 2 = 0 .8 0
60
60 50
40
50
(n m o l/m g /m in ) 30
40 20
10
30 0
-1 0 0 -8 0 -6 0 -4 0 -2 0 0 20
E 1 /2 ( m V )
20
10
0
0 .0 0 0 1 0 .0 0 1 0 .0 1 0 .1 1
[M e ta b o lite ] m M
Microsomes (0.5 mg/mL protein) were preincubated with acivicin (10 m M) for 15 min and then incubated with various
concentrations of either phenol ( , dashed line), HQ ( ), 2-(GS-yl)HQ ( ),2,5-bis-(GS-yl)HQ ( , dashed line), BGHQ
( ), or TGHQ ( ), in the presence of succinoylated cytochrome C (12.5 M) and an NADPH generating system. Superoxide
anion formation is expressed as nmol/mg protein/min. The inset shows the correlation between the oxidation potentials [E1/2
(mV)] for the HQ and its GSH conjuga tes, and their ability to catalyze superoxide anion formation. Each data point represents
the mean ± SEM (n=3).](https://image.slidesharecdn.com/09terrencemonks-121210151658-phpapp02/85/09-terrence-monks-23-320.jpg)