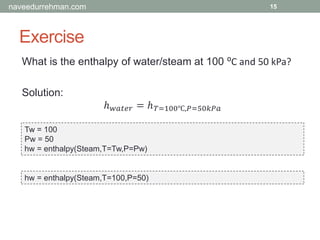
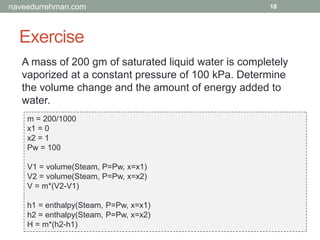
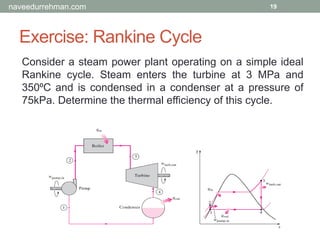
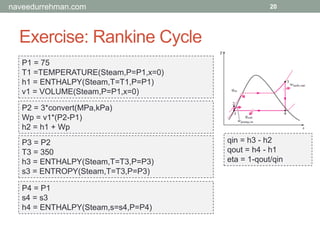
The document provides an overview of the Engineering Equation Solver (EES) software, including installation information, usage examples, and exercises related to thermodynamic problems. It covers solving linear equations, performing unit conversions, and calculating properties of steam and water in various scenarios. Additionally, it includes examples on the Rankine cycle and details on built-in functions for thermodynamic calculations.


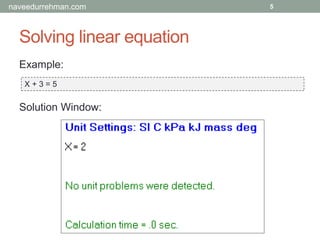
![Solving linear equation
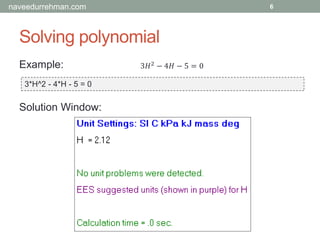
X + 3 = 5
Example:
To obtain solution:
[Menu] Calculate > Solve
or
Press “F2” key
4naveedurrehman.com](https://image.slidesharecdn.com/ees-thermodynamics-170529211421/85/EES-for-Thermodynamics-4-320.jpg)


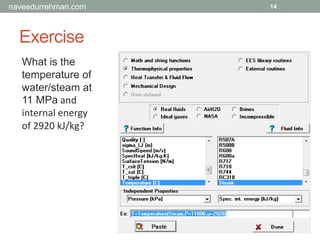
![Built-in Functions
13
To access built-in functions:
[Menu] Options > Function Info
naveedurrehman.com](https://image.slidesharecdn.com/ees-thermodynamics-170529211421/85/EES-for-Thermodynamics-13-320.jpg)