Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Light sources for atomic absorption spectroscopy (aas)

Light sources for atomic absorption spectroscopy (aas)
Optical band gap measurement by diffuse reflectance spectroscopy (drs)

Optical band gap measurement by diffuse reflectance spectroscopy (drs)
TOP-DOWN AND BOTTOM-UP APPROACH IN SYNTHESIS OF NANOPARTICLES.pptx

TOP-DOWN AND BOTTOM-UP APPROACH IN SYNTHESIS OF NANOPARTICLES.pptx
Viewers also liked
Viewers also liked (19)
Introducing the SA-9600 Flowing Gas BET Surface Area Analyzer

Introducing the SA-9600 Flowing Gas BET Surface Area Analyzer
Mate 280 characterization of powders and porous materials

Mate 280 characterization of powders and porous materials
Principles & Applications of Adsorption by Activated Carbon Workshop

Principles & Applications of Adsorption by Activated Carbon Workshop
Proposing a new empirical adsorption isotherm known as Adejo-Ekwenchi isotherm

Proposing a new empirical adsorption isotherm known as Adejo-Ekwenchi isotherm
Adsorption Isotherm Parameters of CH4 and CO2 on MIL-100 and MIL-101

Adsorption Isotherm Parameters of CH4 and CO2 on MIL-100 and MIL-101
Similar to Bet
Similar to Bet (20)
Bet
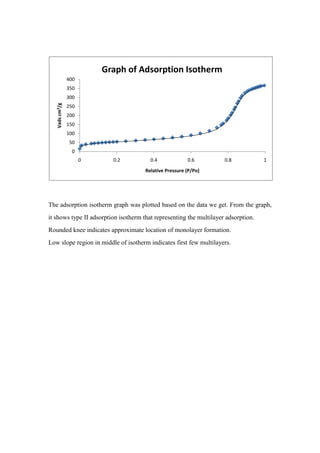
- 1. Graph of Adsorption Isotherm 400 350 300 Vads cm³/g 250 200 150 100 50 0 0 0.2 0.4 0.6 0.8 1 Relative Pressure (P/Po) The adsorption isotherm graph was plotted based on the data we get. From the graph, it shows type II adsorption isotherm that representing the multilayer adsorption. Rounded knee indicates approximate location of monolayer formation. Low slope region in middle of isotherm indicates first few multilayers.
- 2. BET Surface Area BET surface area analysis is a technique used to determine the specific surface area of a material. Model of adsorption extended to multilayers. BET equation: V C ( P P0 ) Vm (1 P P0 )(1 P P0 C ( P P0 ) Or it’s linearized form: 1 1 C 1 P V [( P0 P ) 1] VmC VmC P0 By comparing with y = mx+C y- axis = 1/[V (Po/P)-1] x-axis = P/Po m (slope/gradient) = C (intercept) = Then, graph of 1/[V (Po/P)-1] versus P/Po is plotted. BET Graph 0.008 0.007 y = 0.022x + 4E-05 0.006 1/[V(Po/P - 1)] 0.005 0.004 0.003 0.002 0.001 0 0 0.05 0.1 0.15 0.2 0.25 0.3 0.35 Relative Pressure (P/Po)
- 3. By using Microsoft Excel, we obtain a linear equation y = 0.0229x + 4×10-5. Slope = = 0.0229 (Equation 1) Intercept = = 4×10-5 (Equation 2) By solving equation 1 and 2, C = 573.5 Vm = 43.59 ml/g The specific surface area is equal to monolayer capacity x Avogadro’s constant x cross section of adsorbed molecule, that is Sm = nm × Am × N Sm = nm × Am × N nm = = 0.00195 mol/g Sm = nm × Am × N Sm = 0.00195 mol/g × (16.2 ×10 -20 m2) × (6.023 x 1023 mol-1) Sm = 190.2 m2/g
- 4. Langmuir Model Vads = Or it’s linearized form, By comparing with y = mx+C y- axis = p/Vad x-axis = p m (slope/gradient) = C (intercept) = Then, graph of p/Vad versus p is plotted. Langmuir Graph 4.5 4 y = 0.016x + 0.209 3.5 3 P/Vad 2.5 2 1.5 1 0.5 0 0 50 100 150 200 250 P
- 5. By using Microsoft Excel, we obtain a linear equation y = 0.0166x + 0.2094 Slope = = 0.0166 (Equation 1) Intercept = = 0.2094 (Equation 2) By solving equation 1, Vm = 60.2 ml/g The specific surface area is equal to monolayer capacity x Avogadro’s constant x cross section of adsorbed molecule, that is Sm = nm × Am × N Sm = nm × Am × N nm = = 0.00269 mol/g Sm = nm × Am × N Sm = 0.00269 mol/g × (16.2 ×10 -20 m2) × (6.023 x 1023 mol-1) Sm =262.3 m2/g