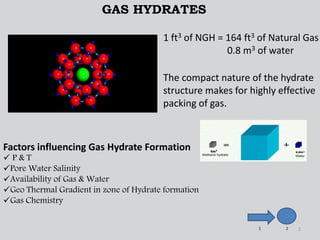
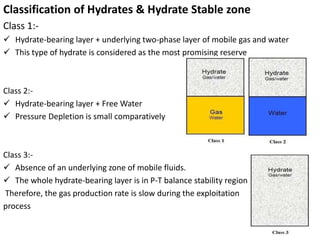
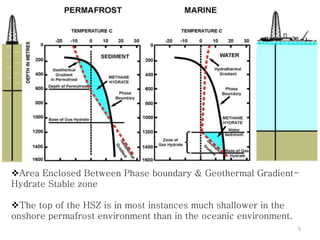
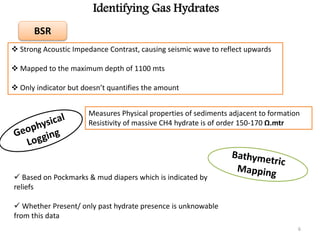
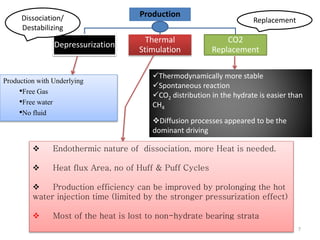
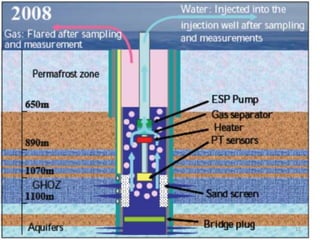
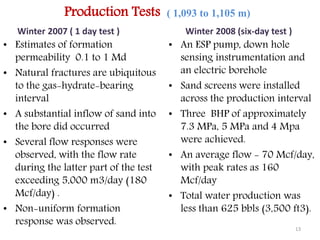
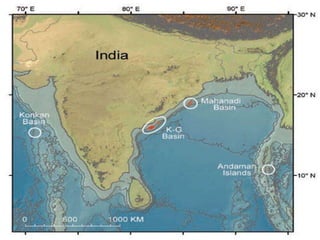
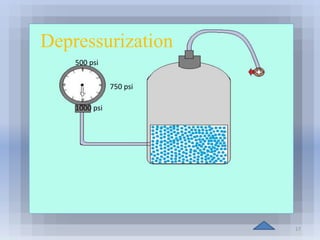
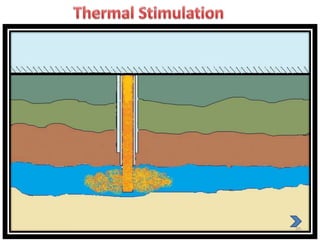
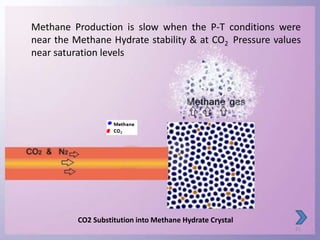
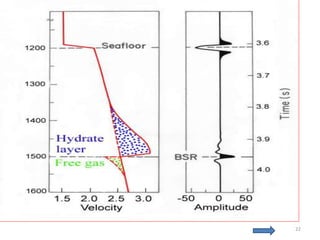
Natural gas hydrates contain large quantities of methane trapped within ice crystal structures. Exploring and producing natural gas hydrates faces challenges related to their compact structure, formation factors, and location within stability zones. Initial production tests at the Mallik gas field involved depressurization and achieved flow rates up to 160 Mcf/day with minimal water production, demonstrating the potential for natural gas hydrate production but also issues like sand ingress. Replacing methane with carbon dioxide offers an alternative production method due to CO2's more favorable thermodynamic properties and easier distribution within the hydrate crystal structure.