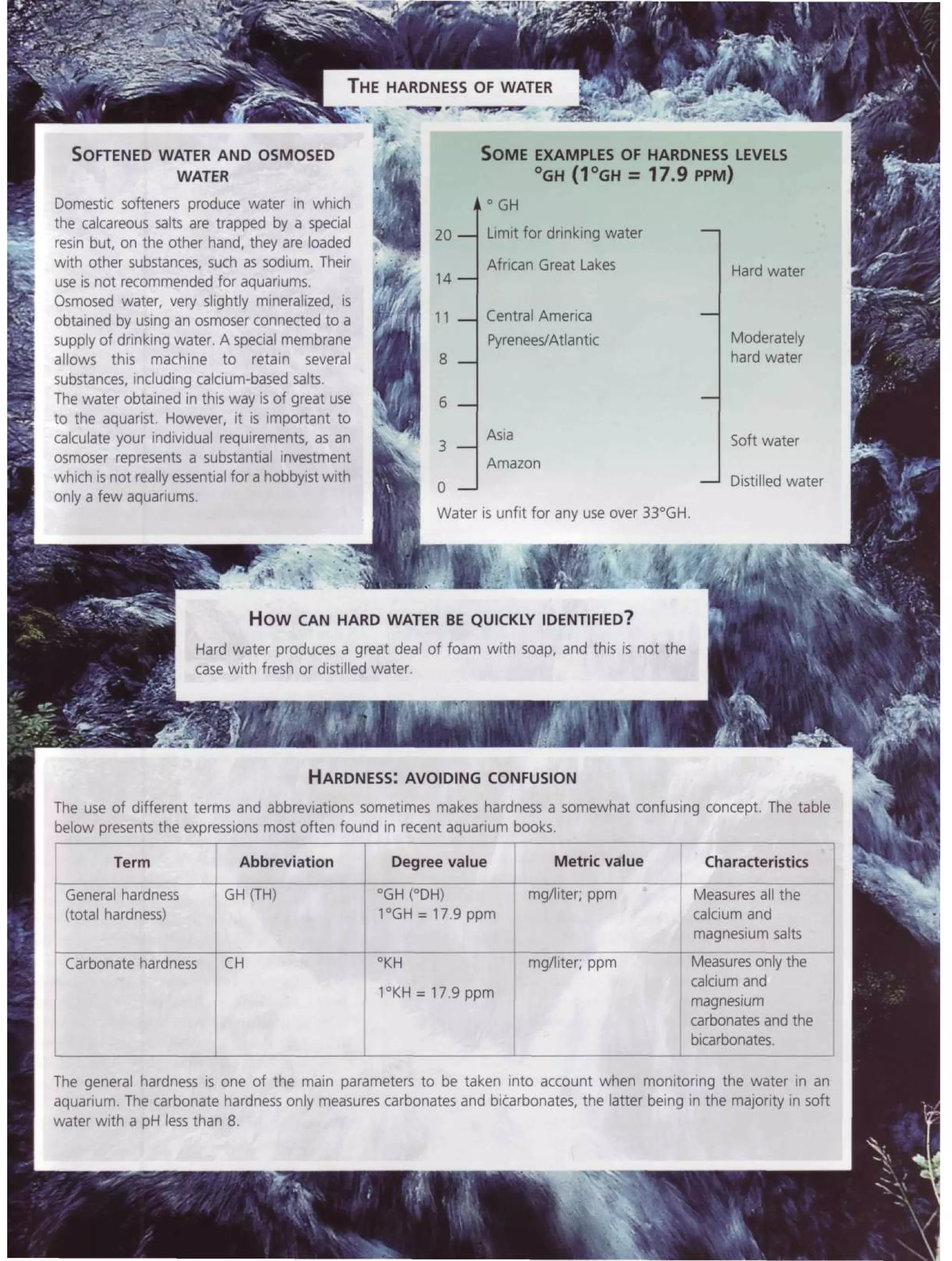
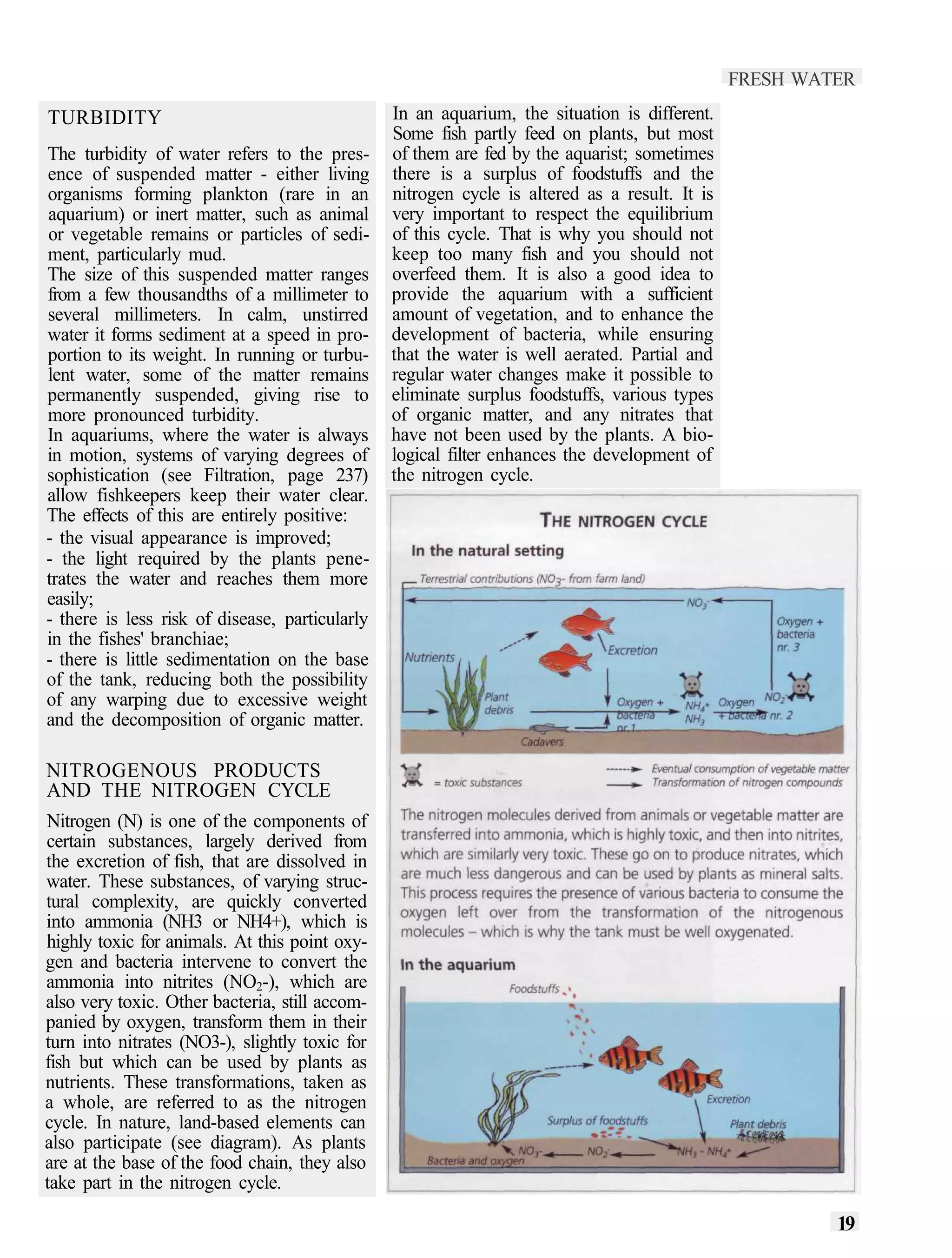
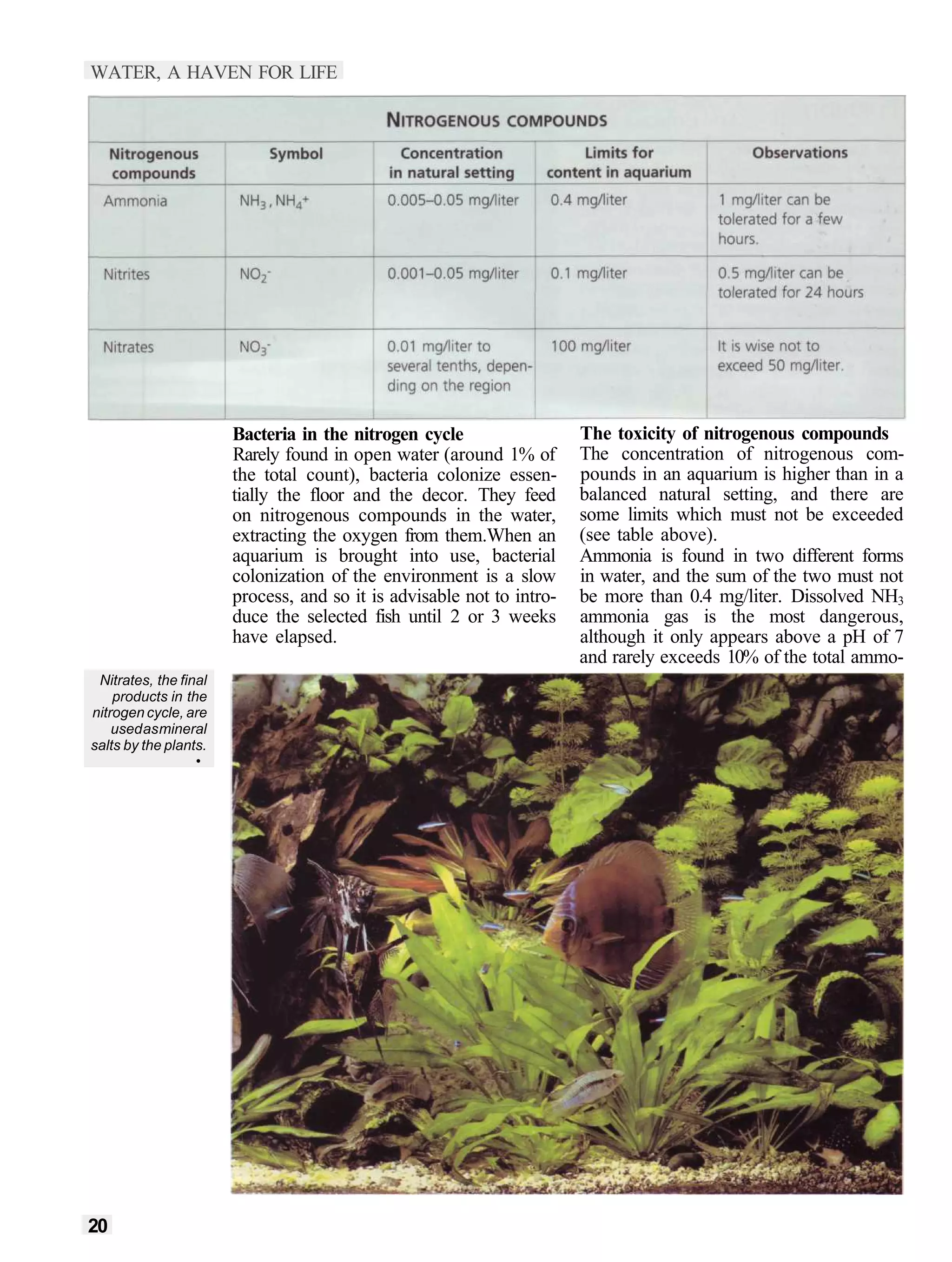
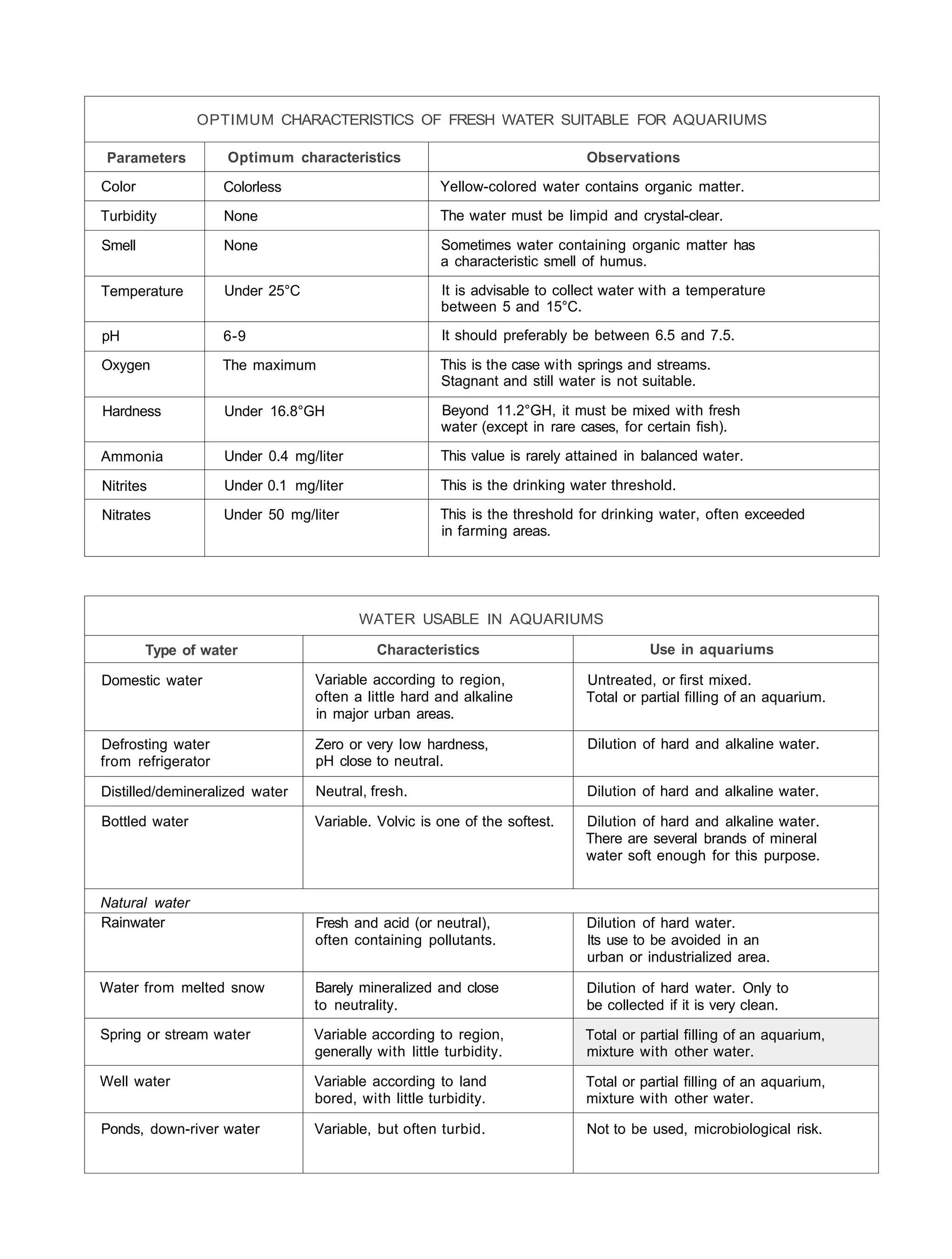
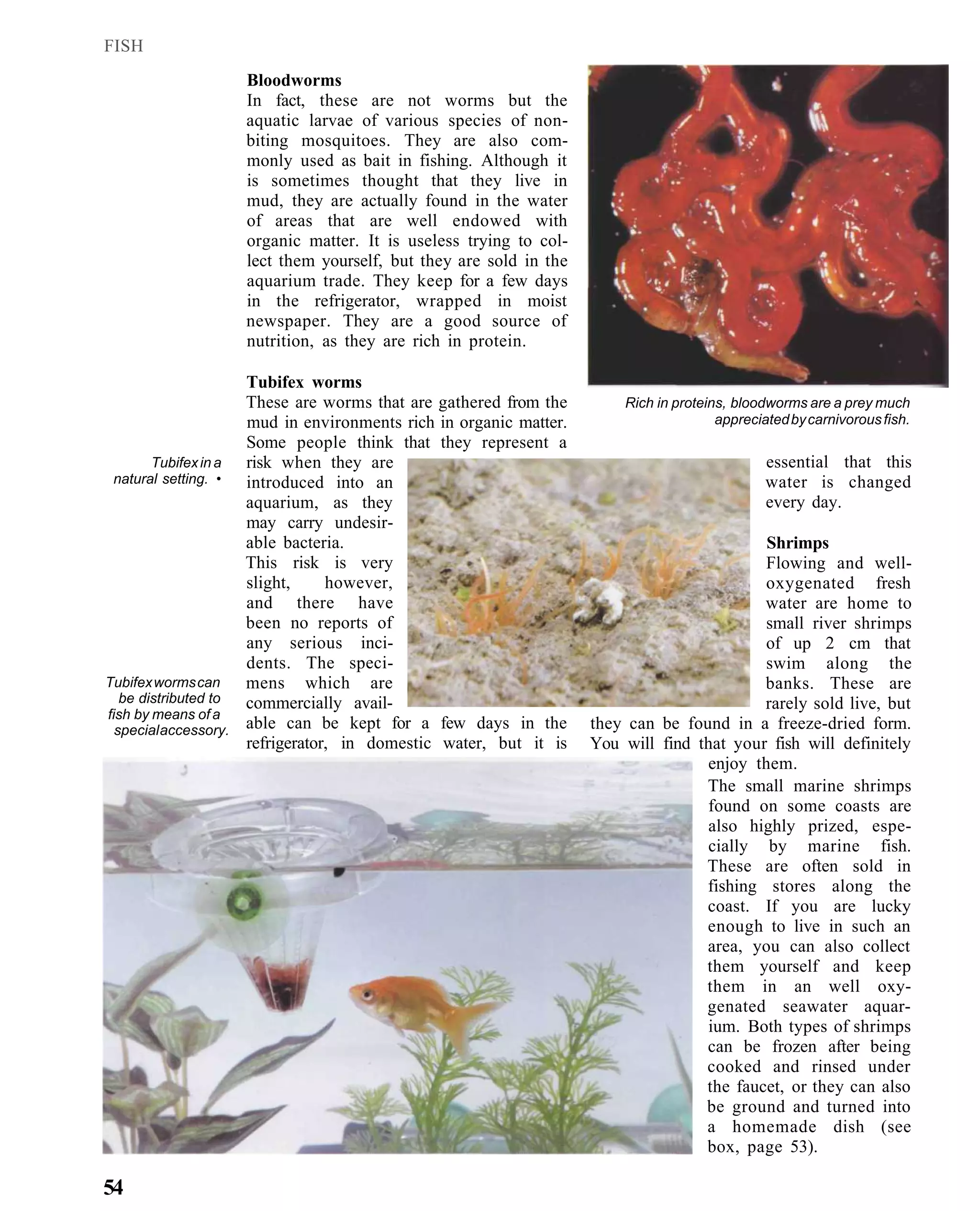
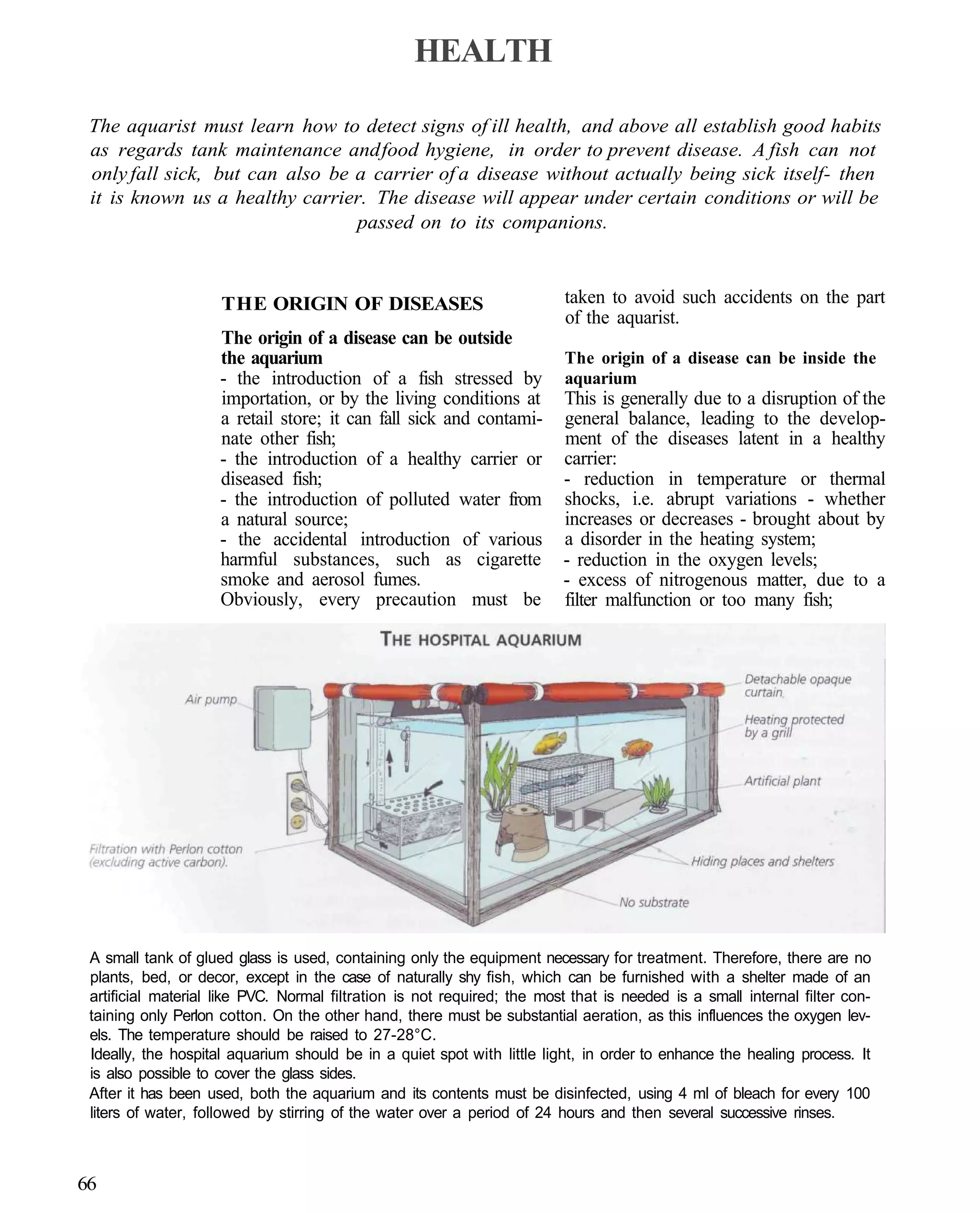
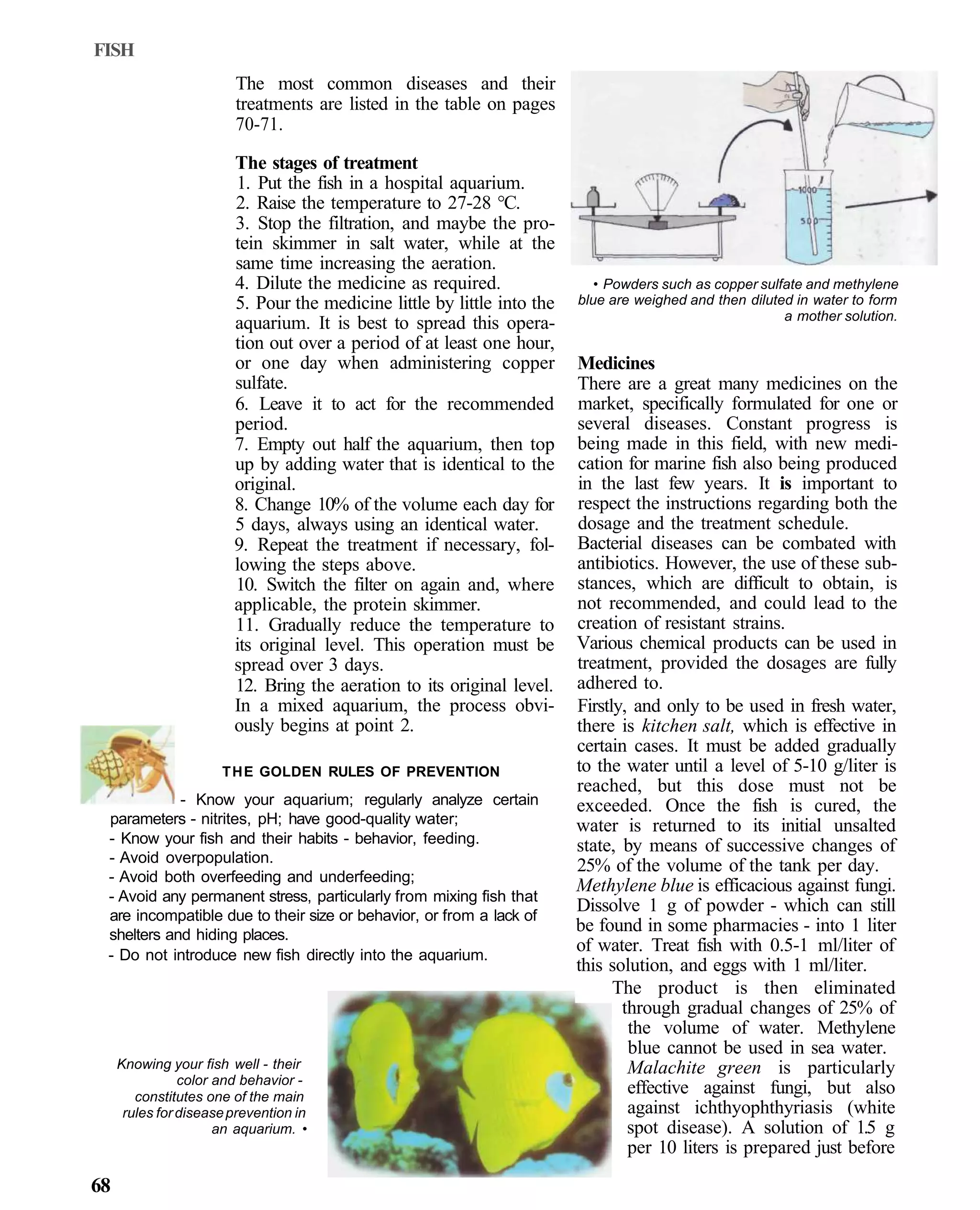
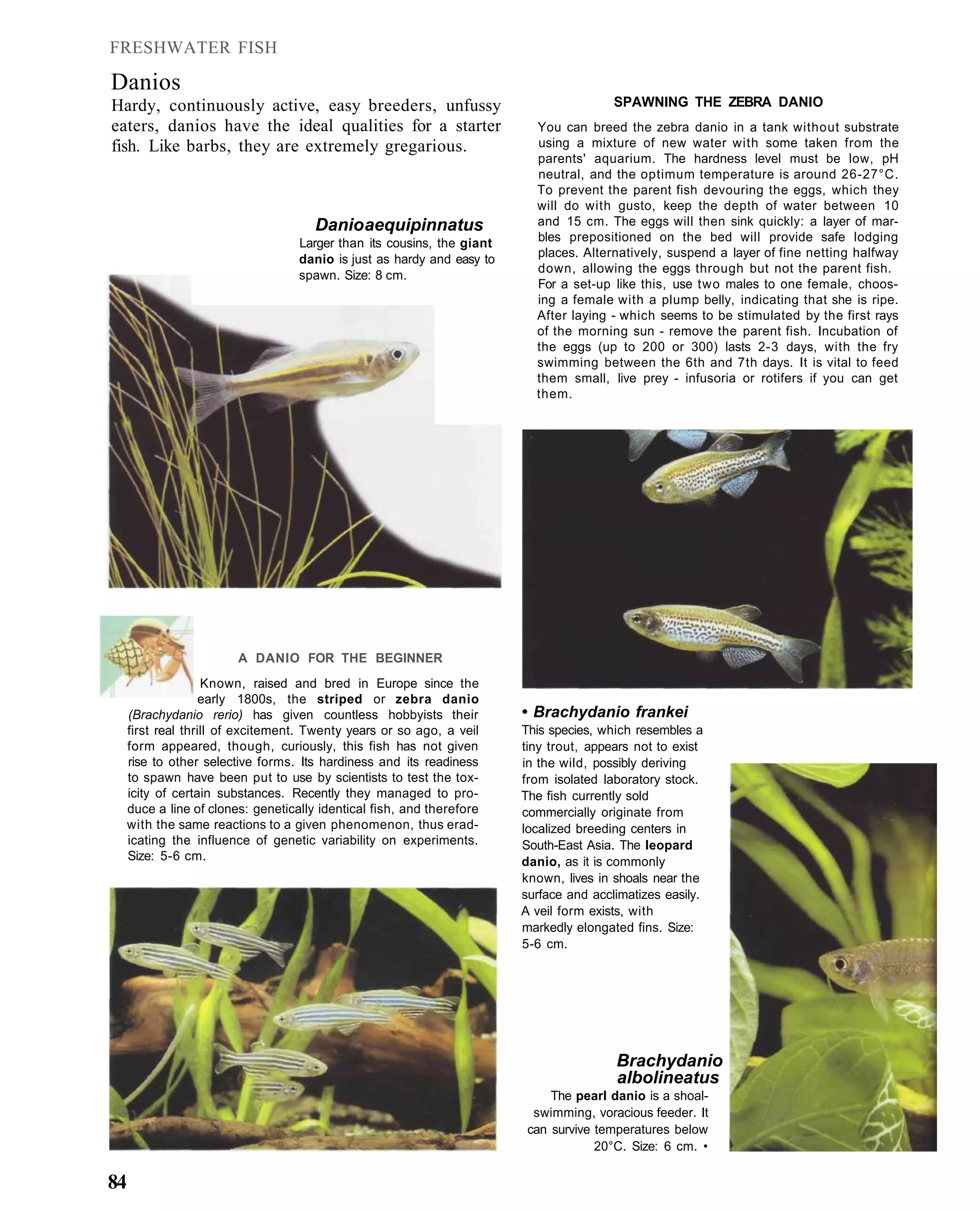
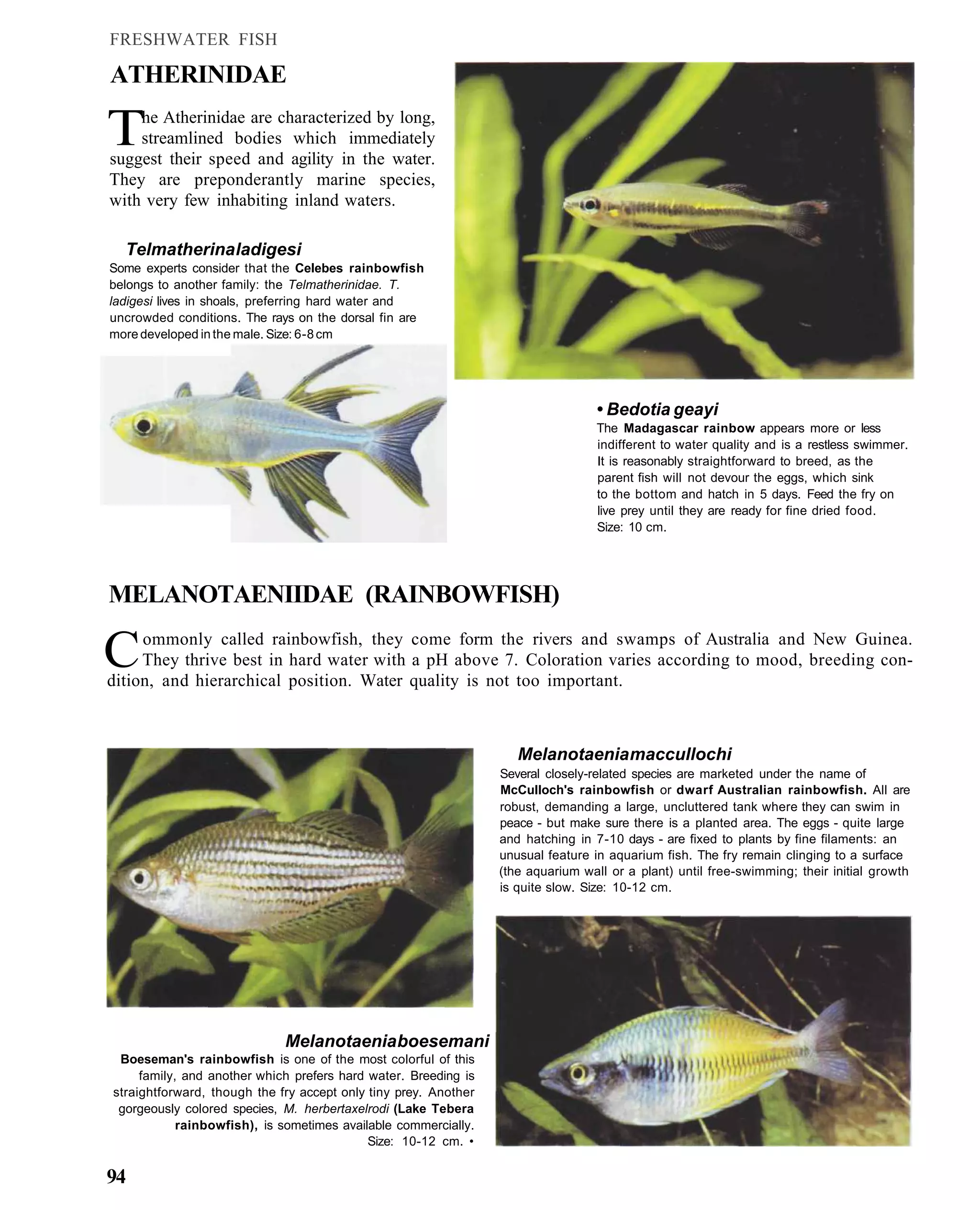
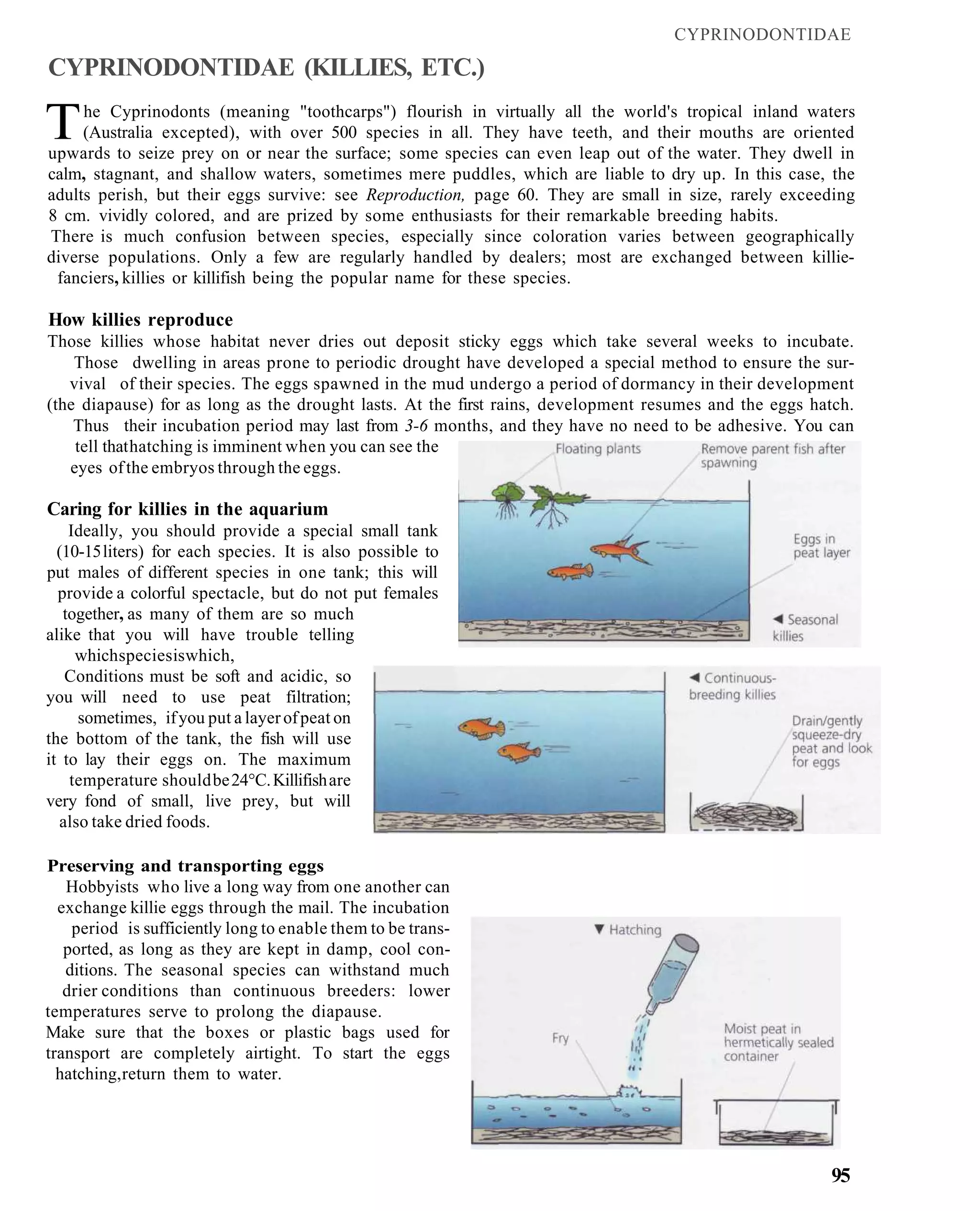
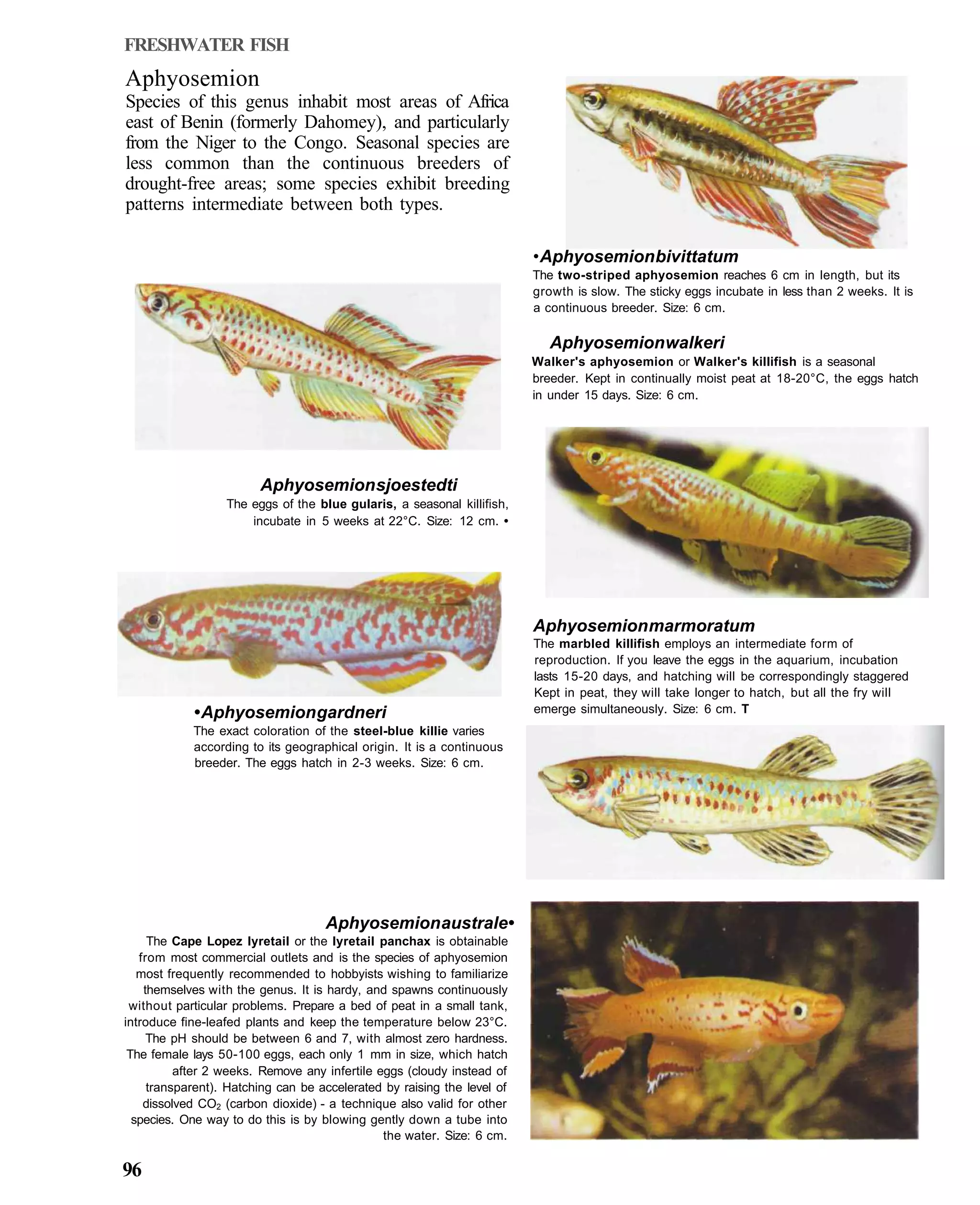
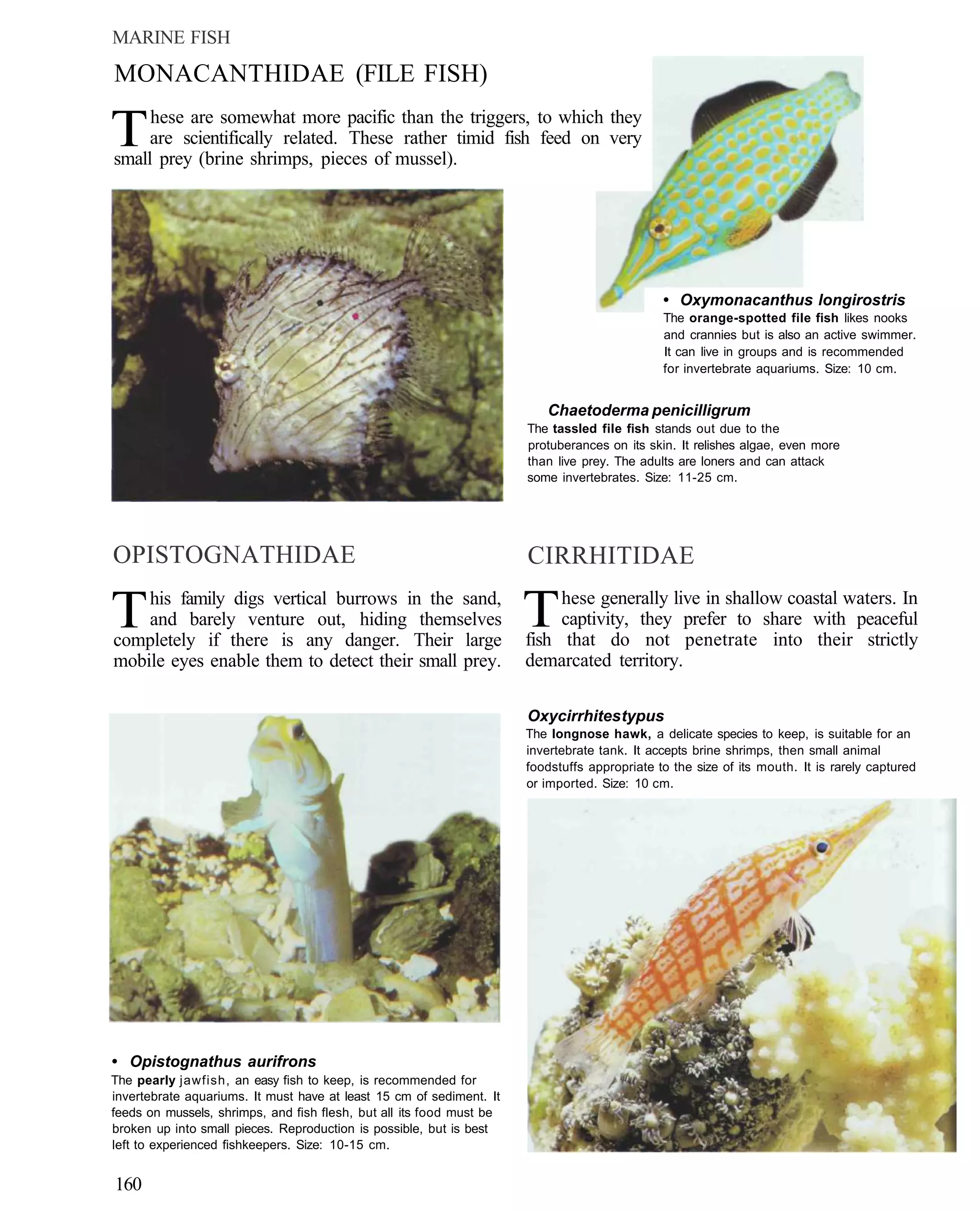
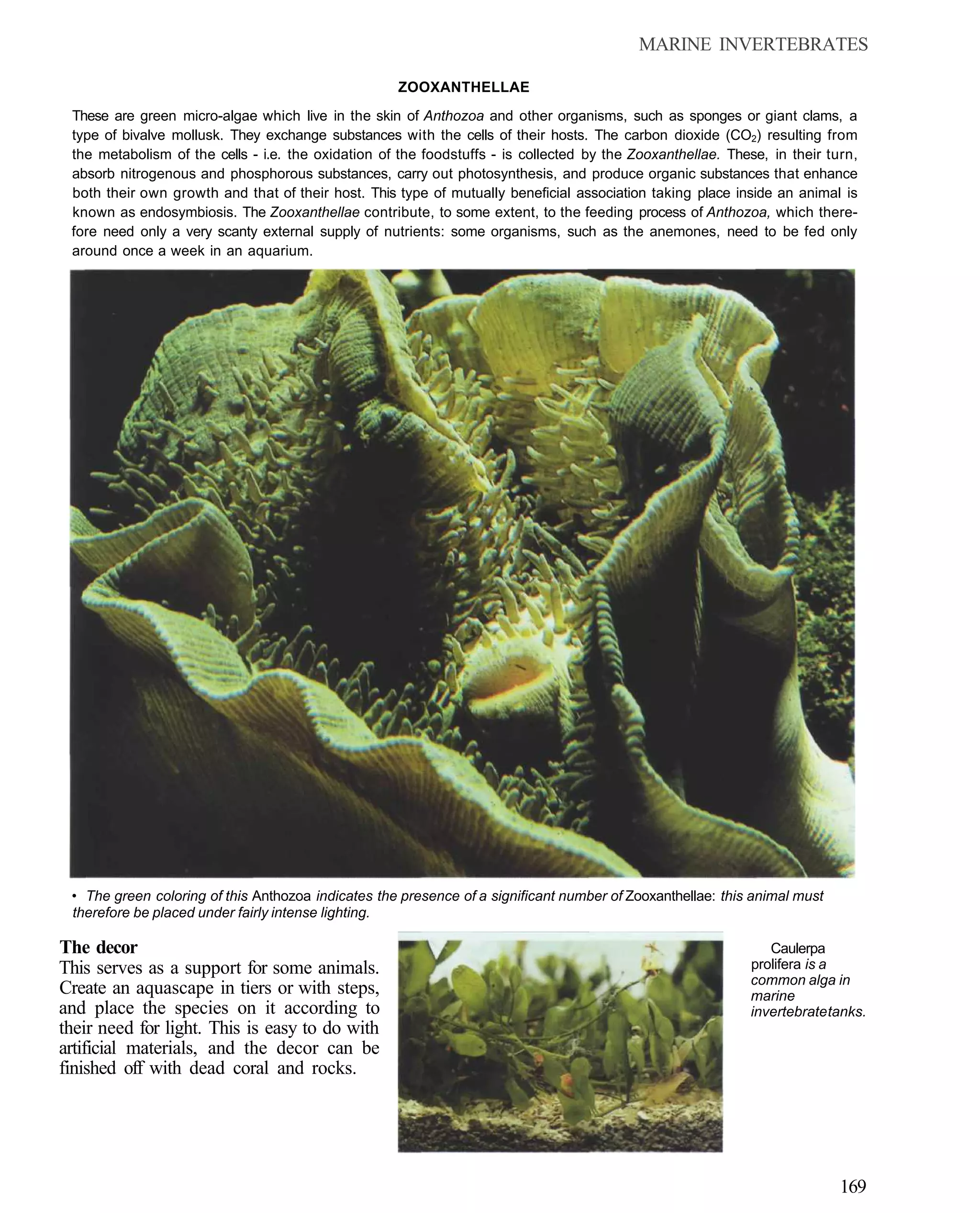
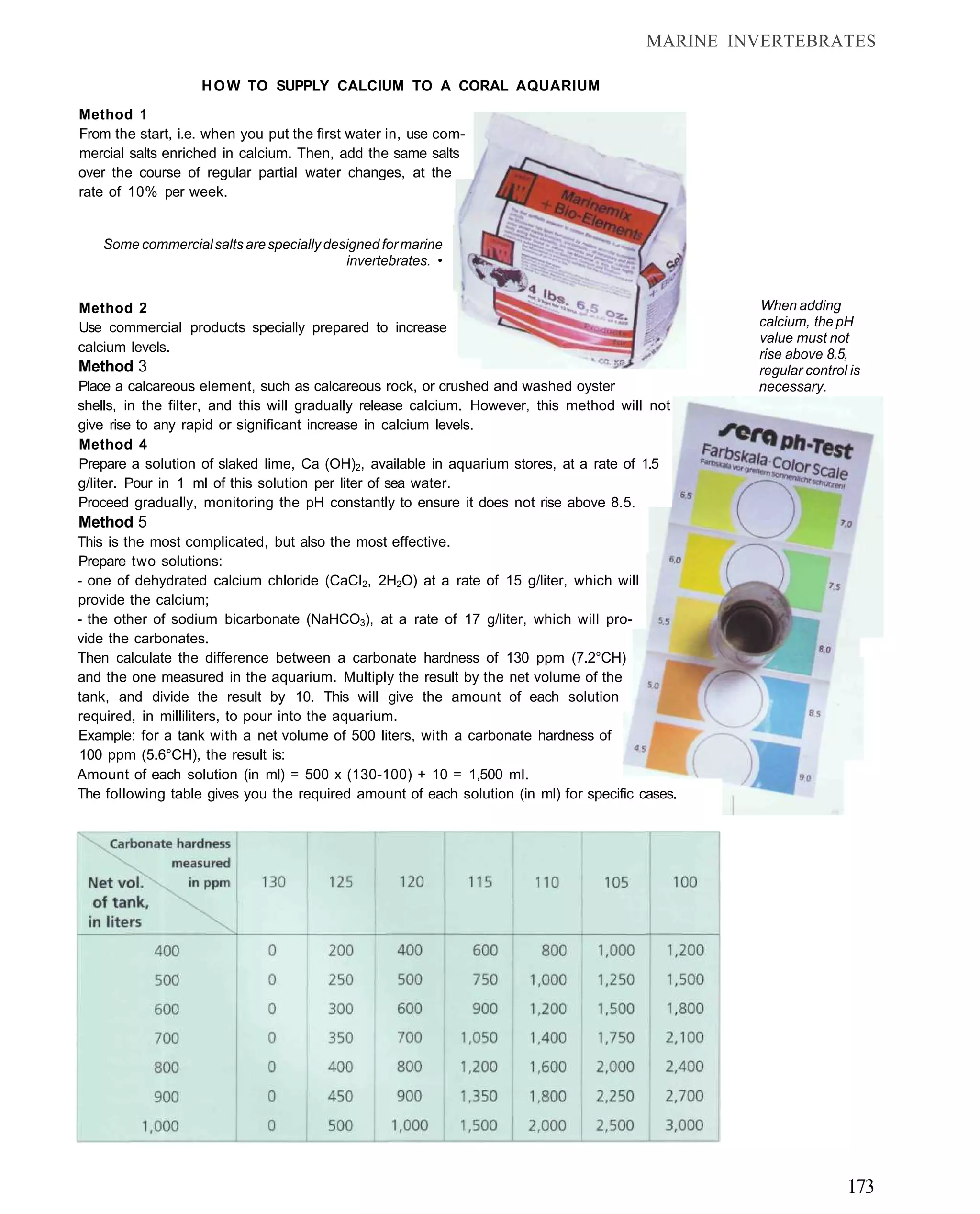
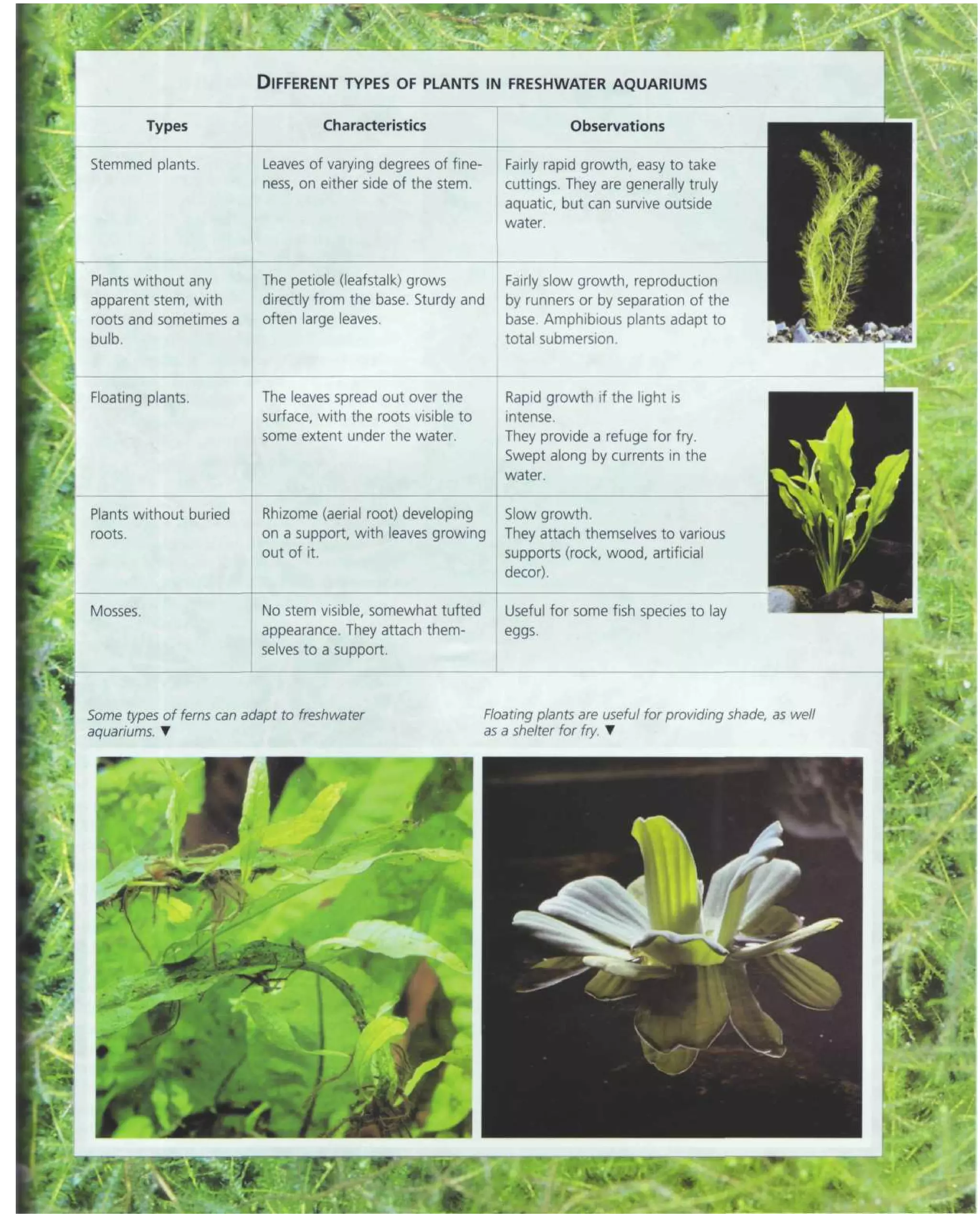
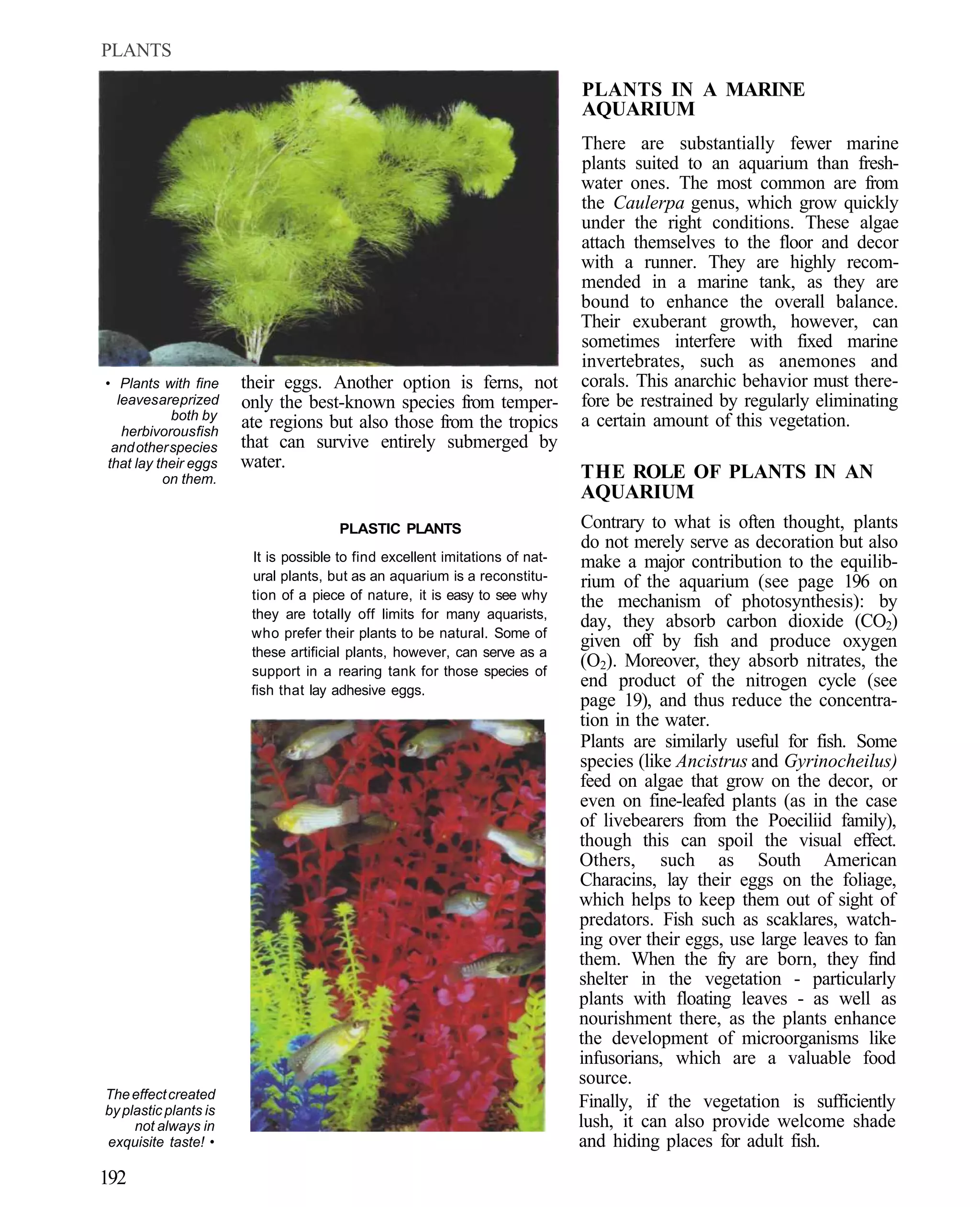
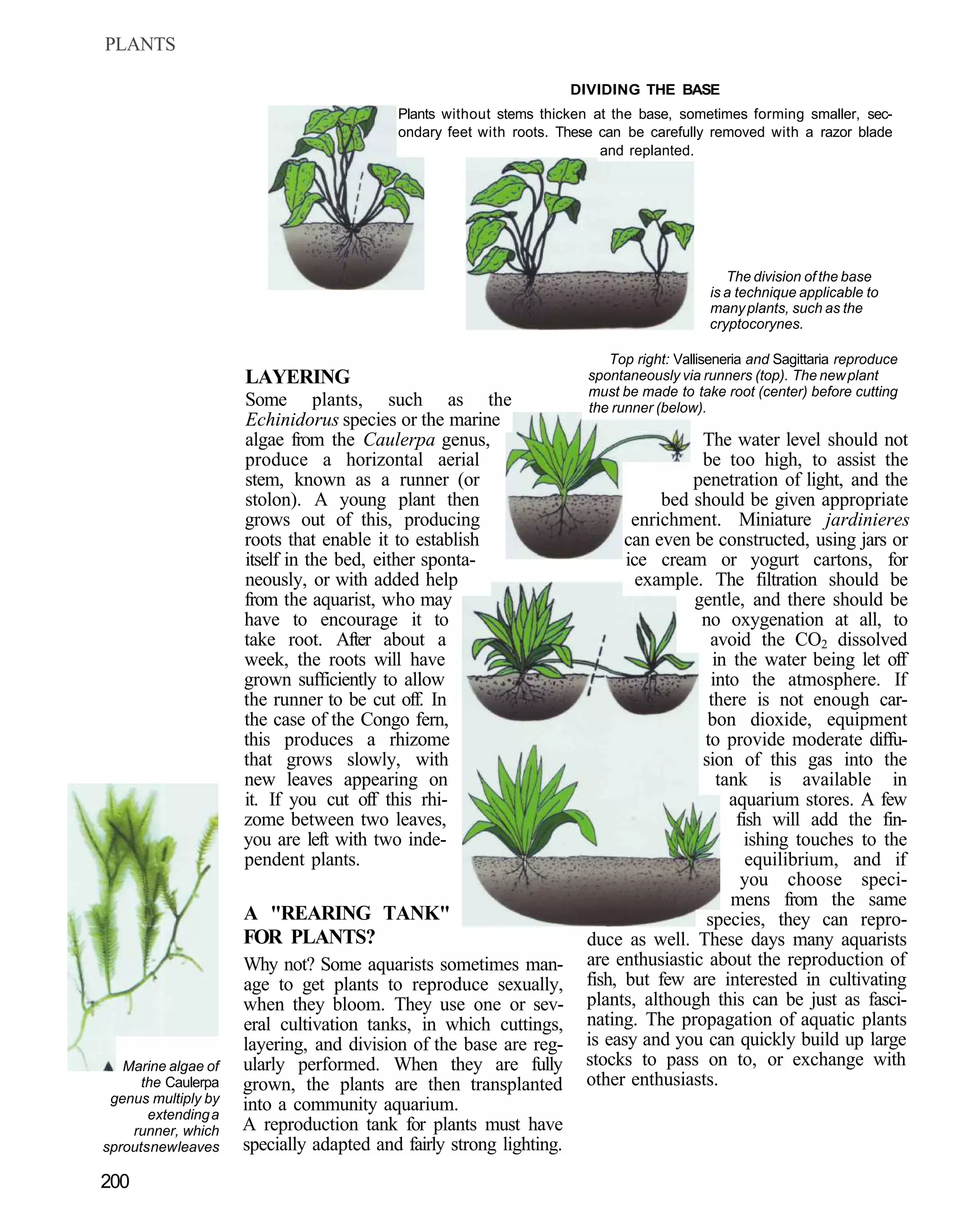
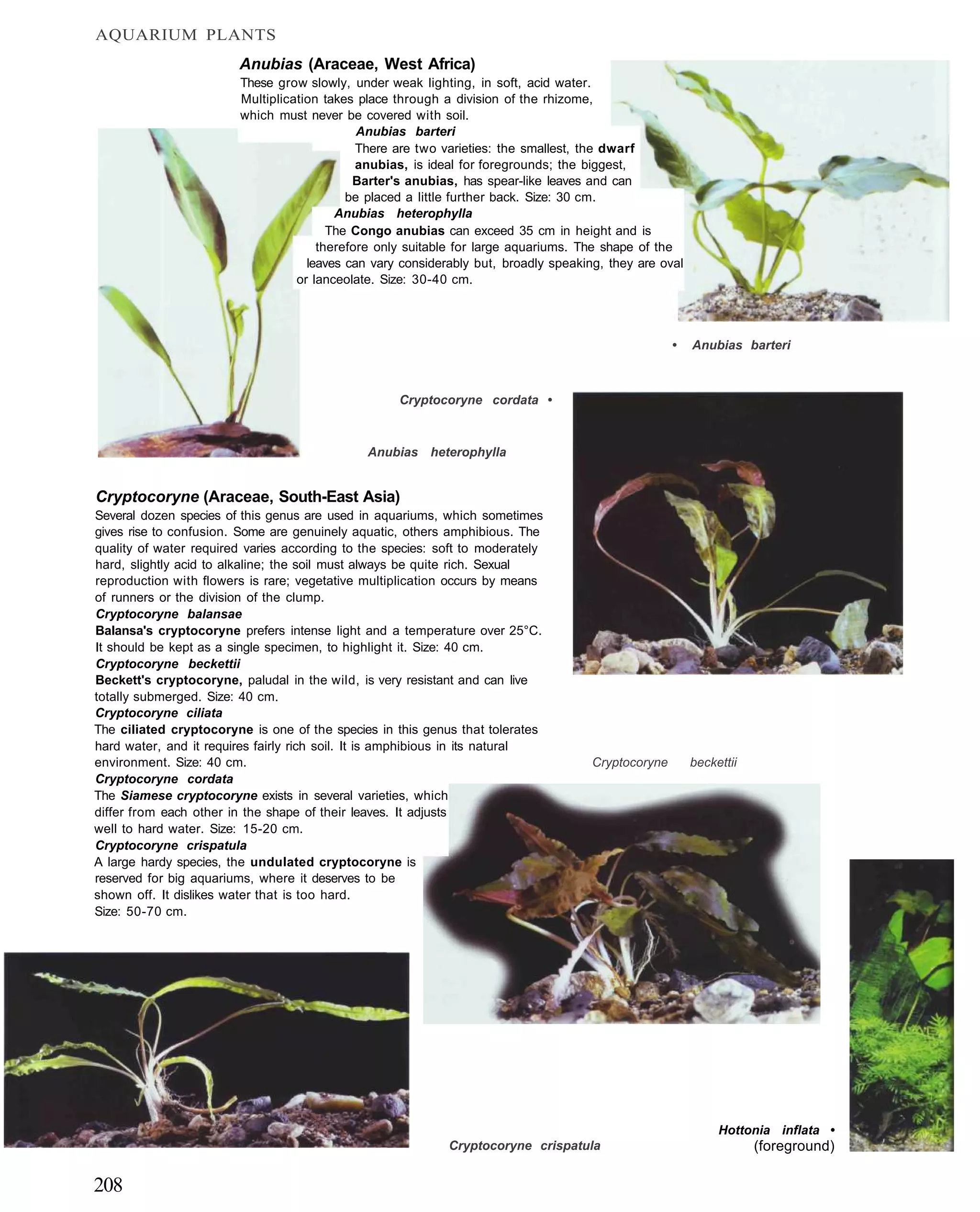
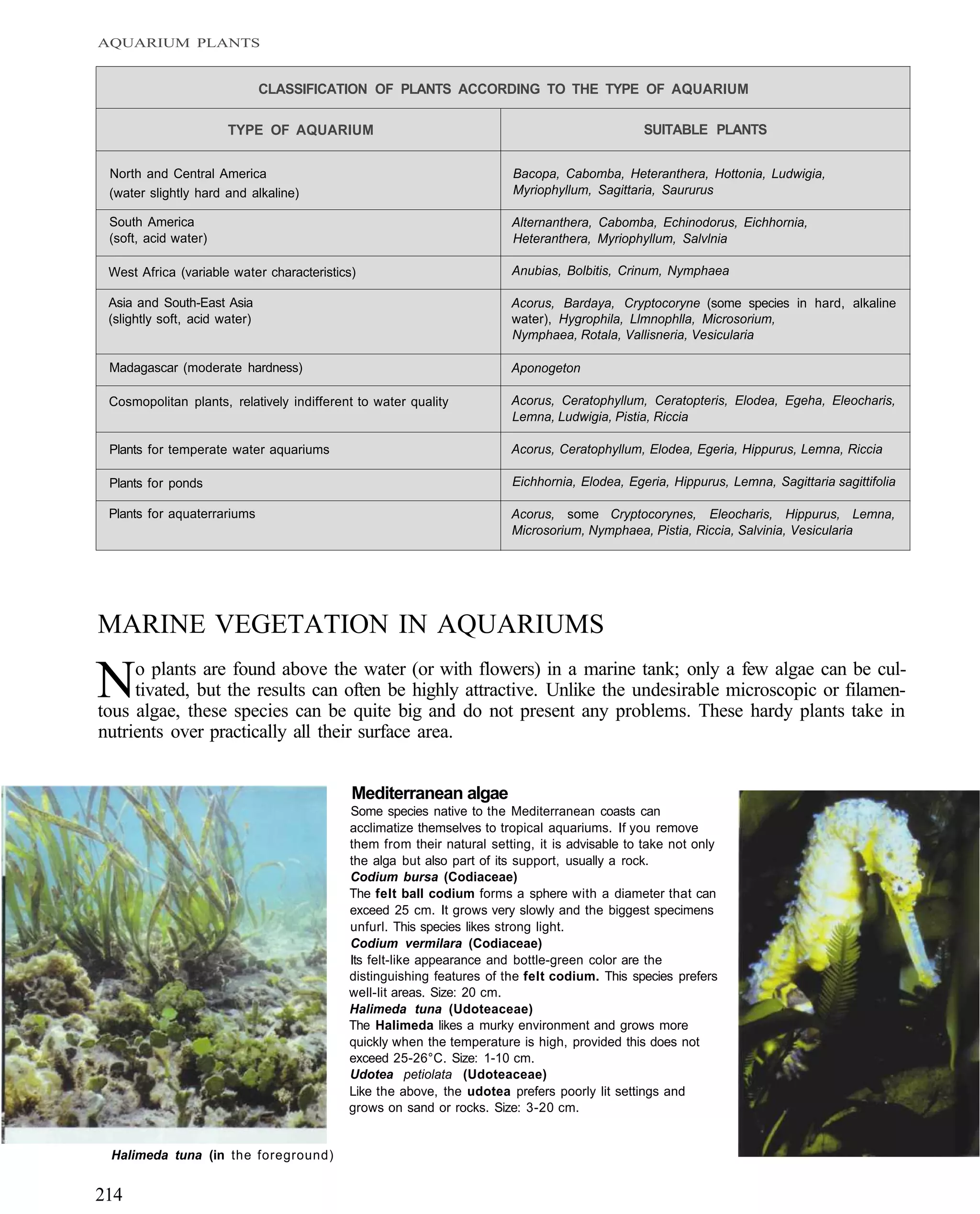
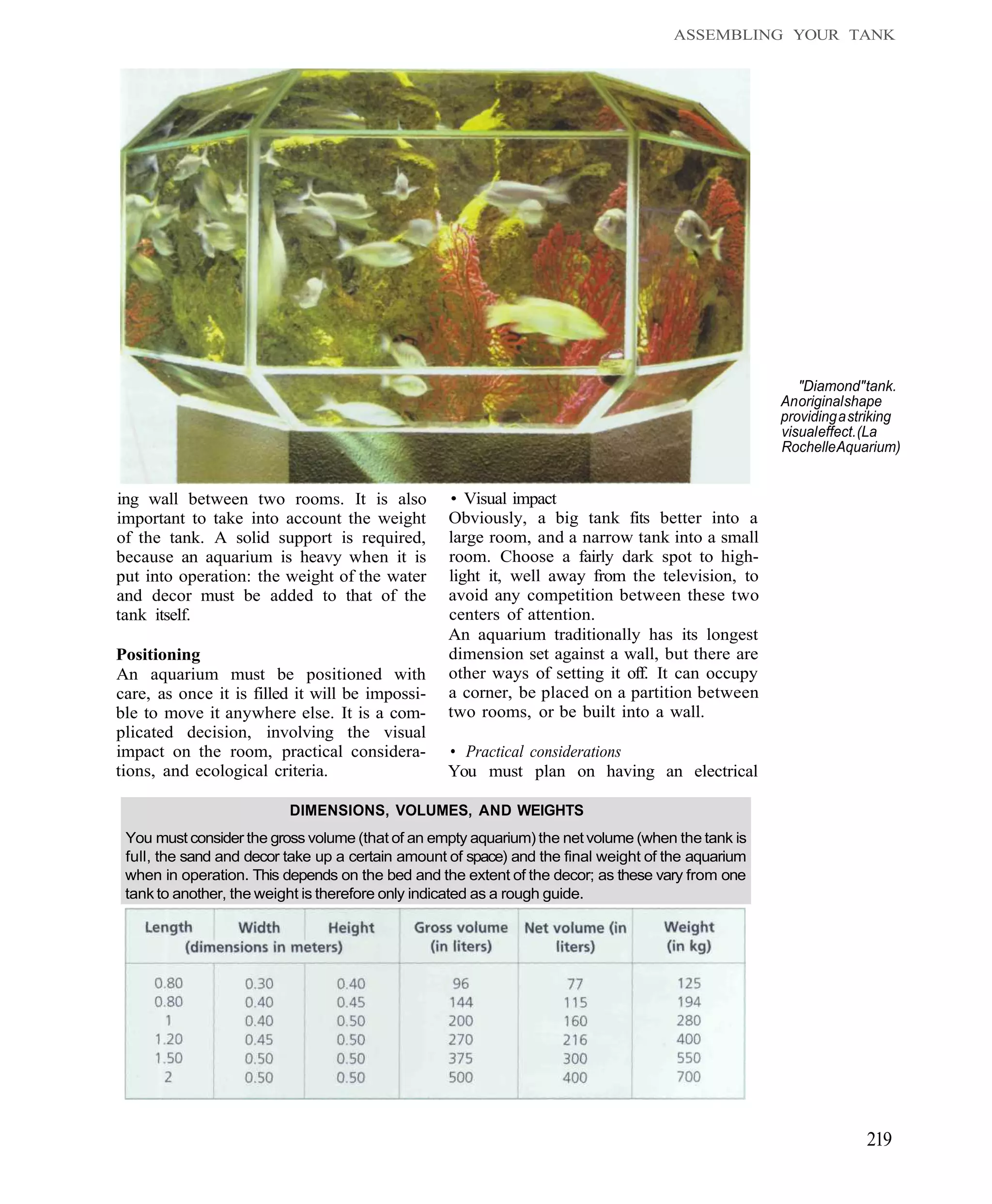
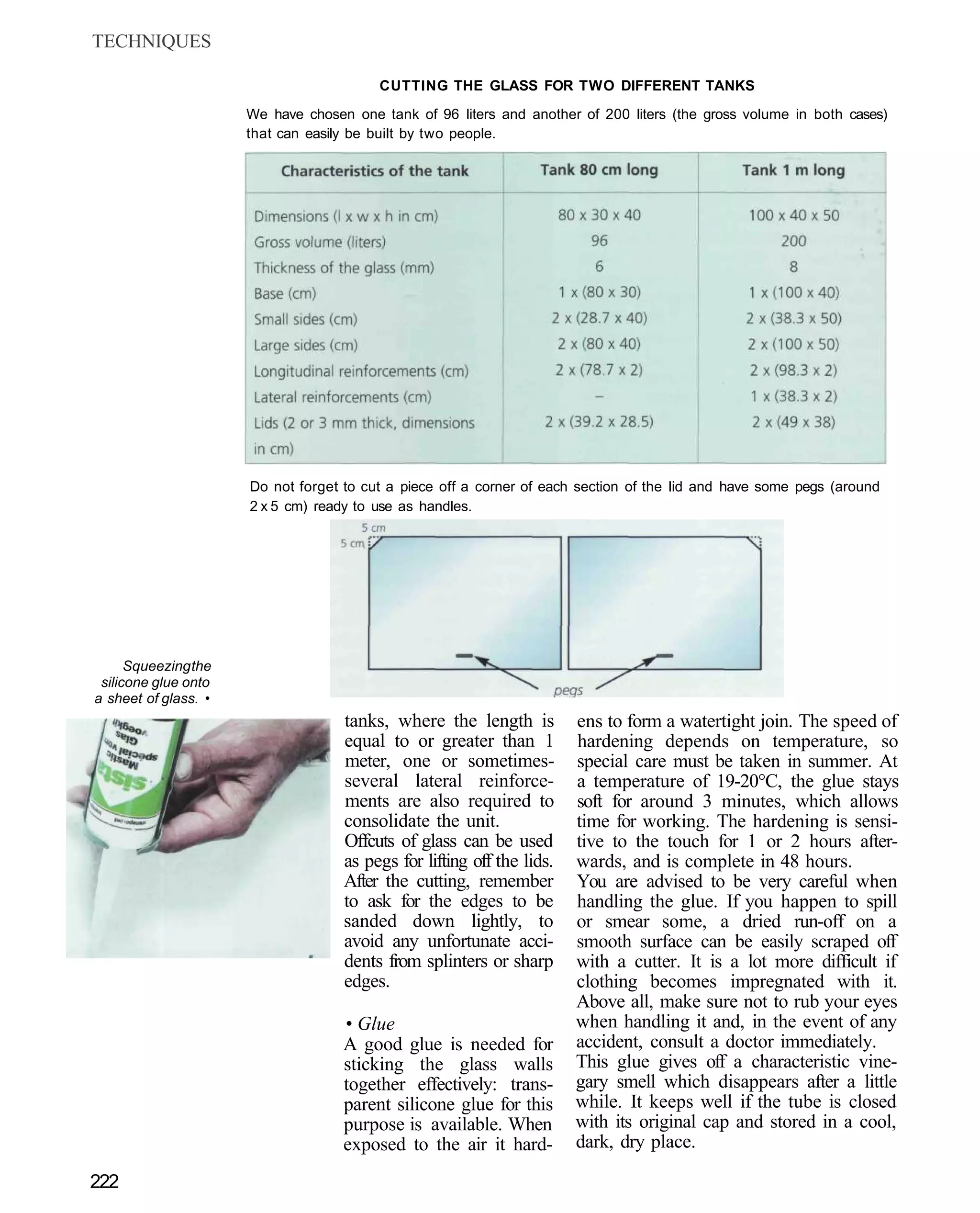
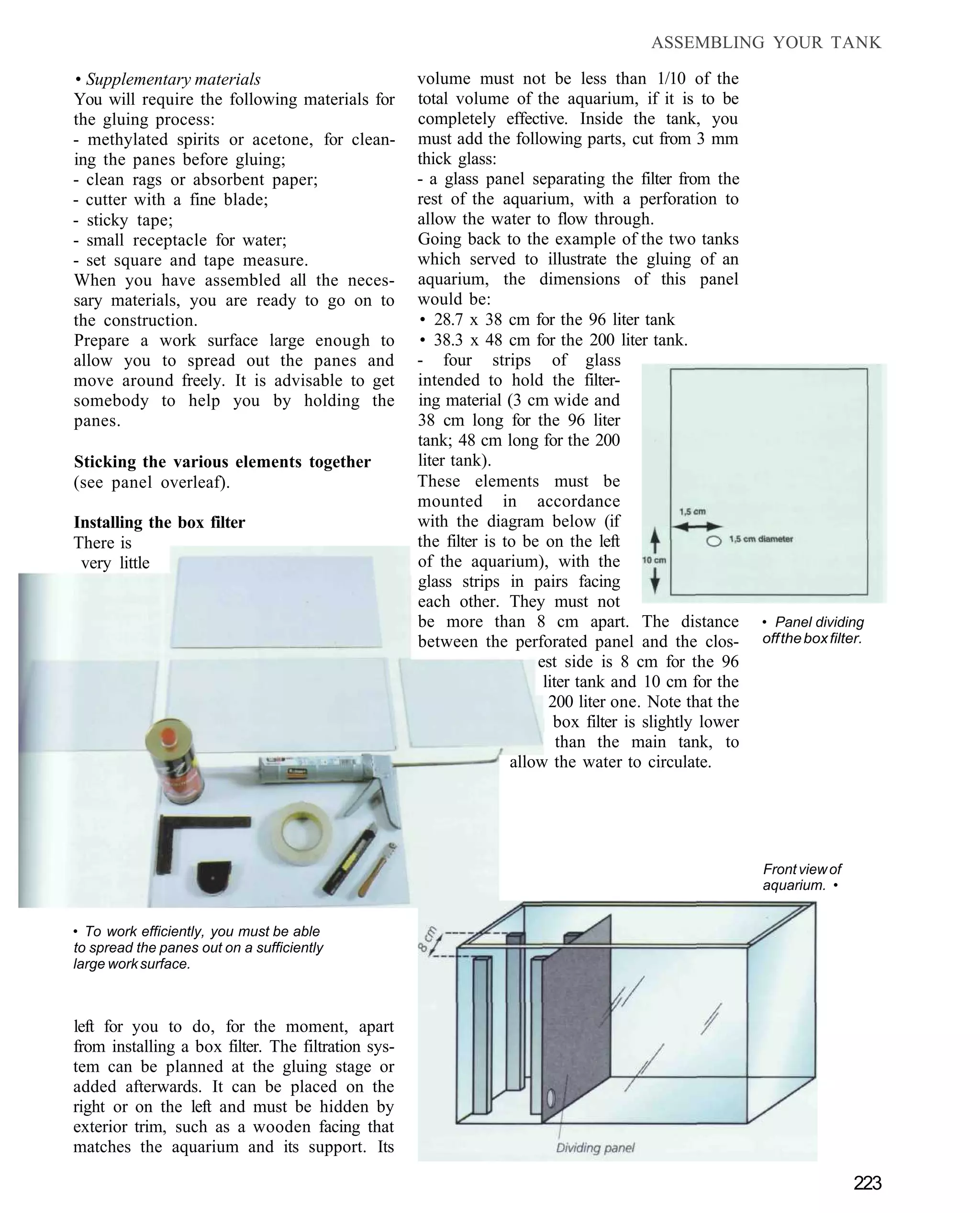
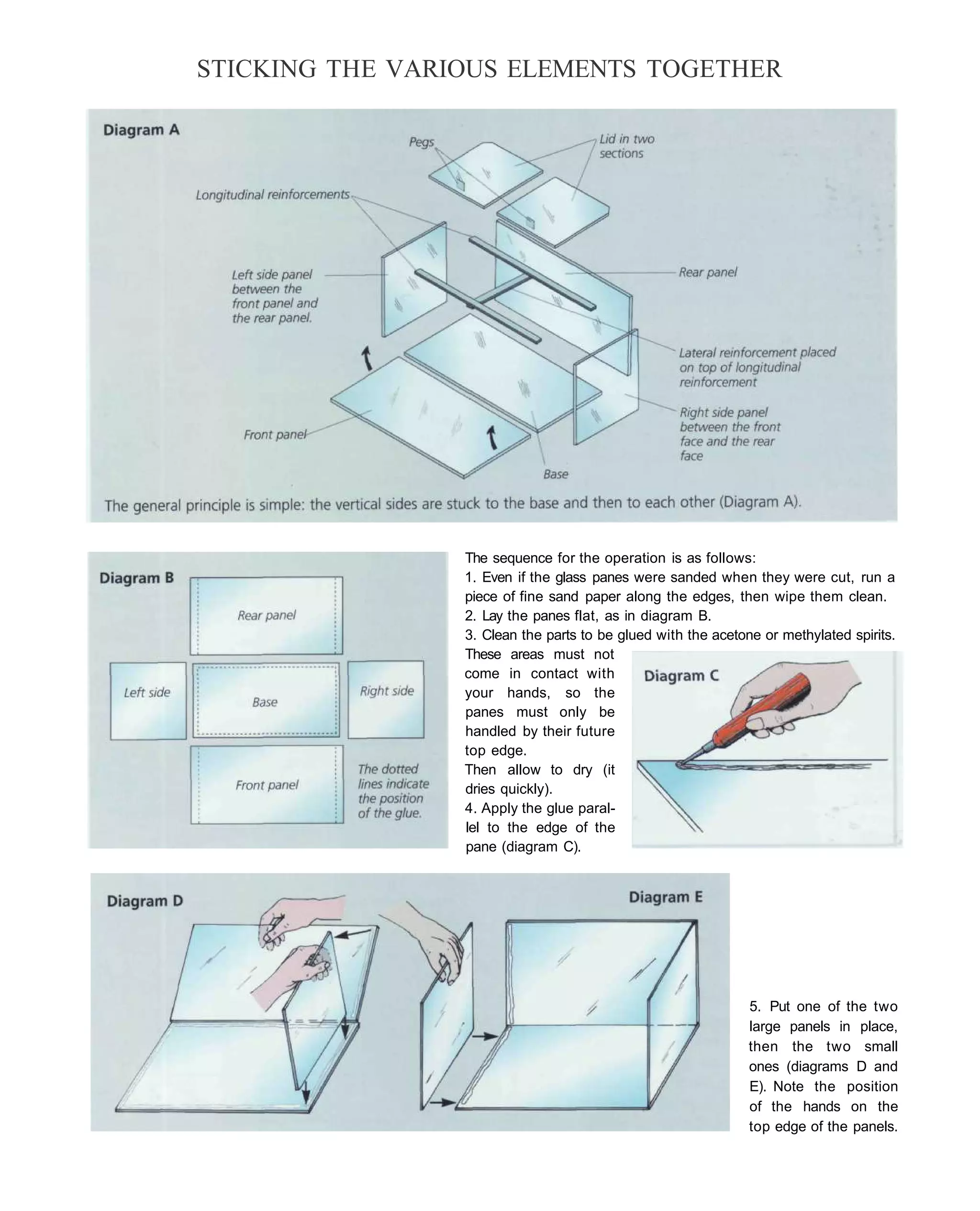
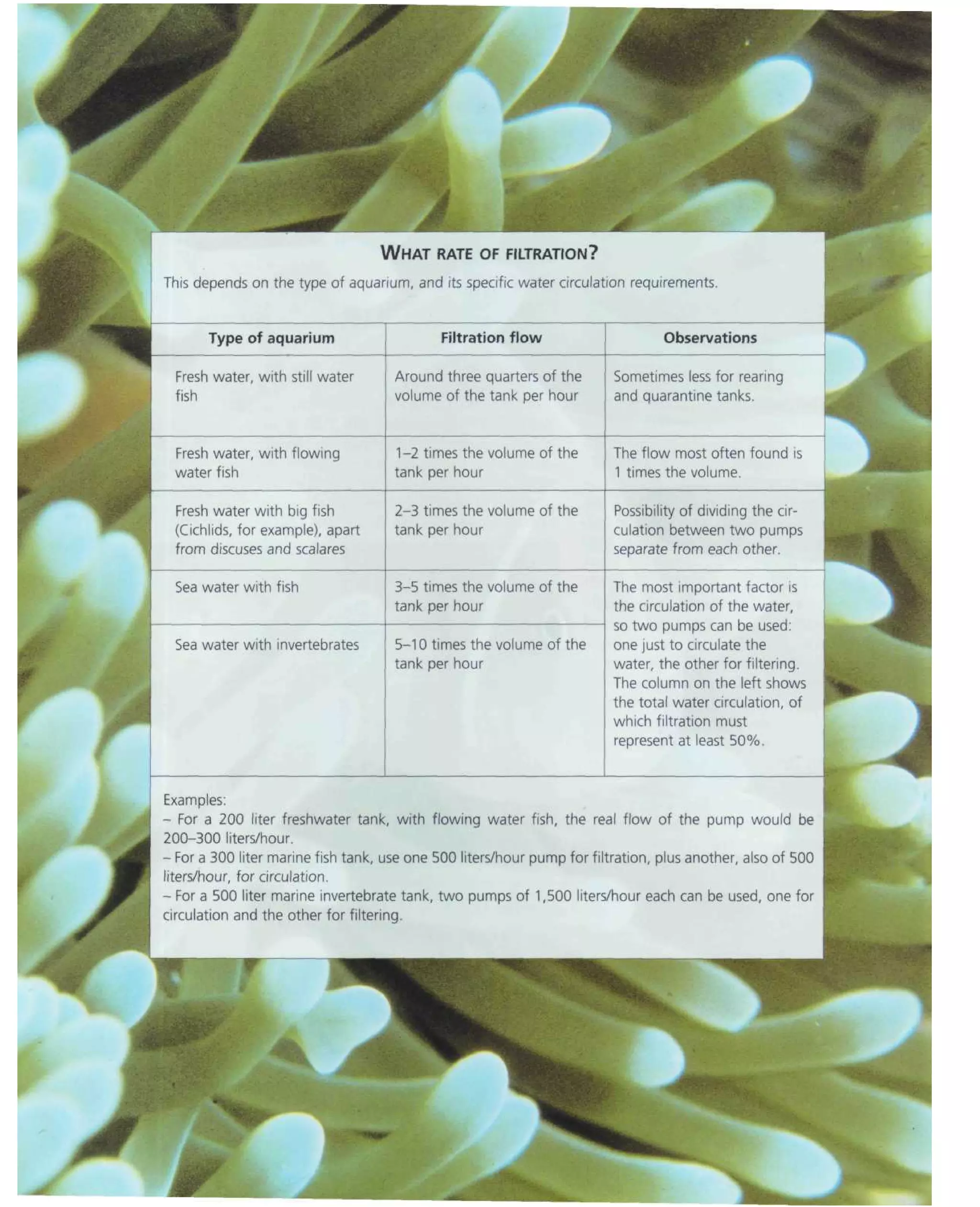
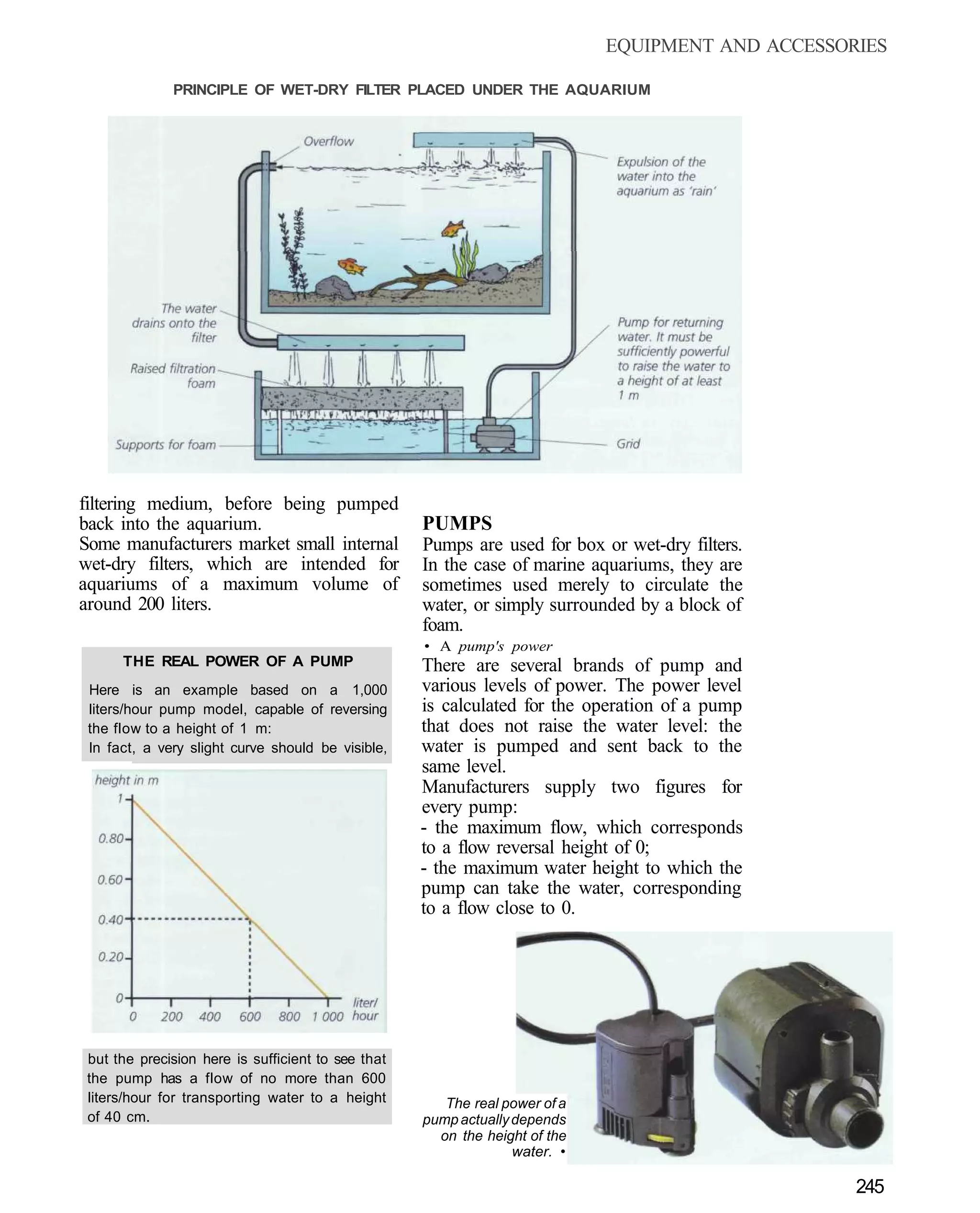
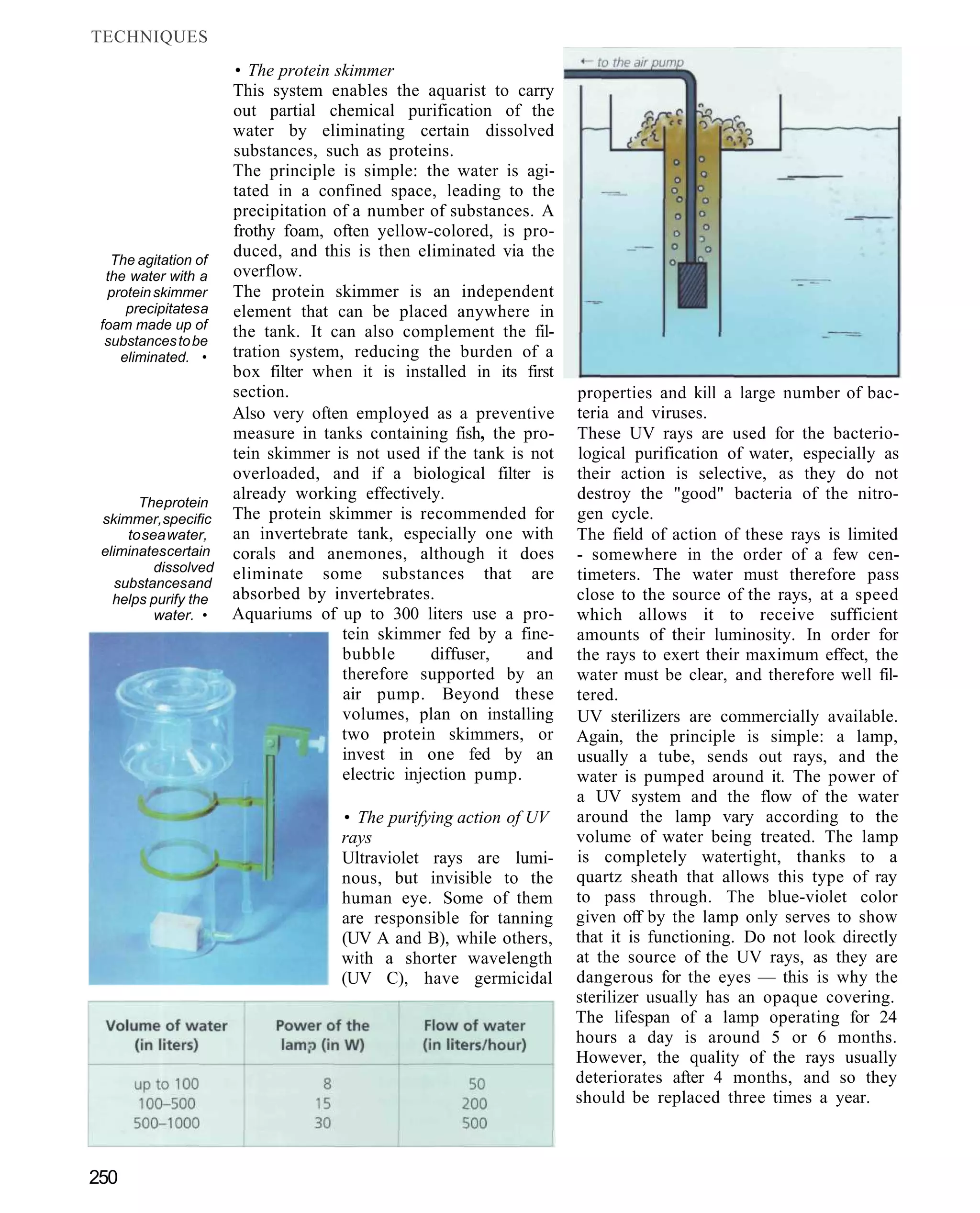
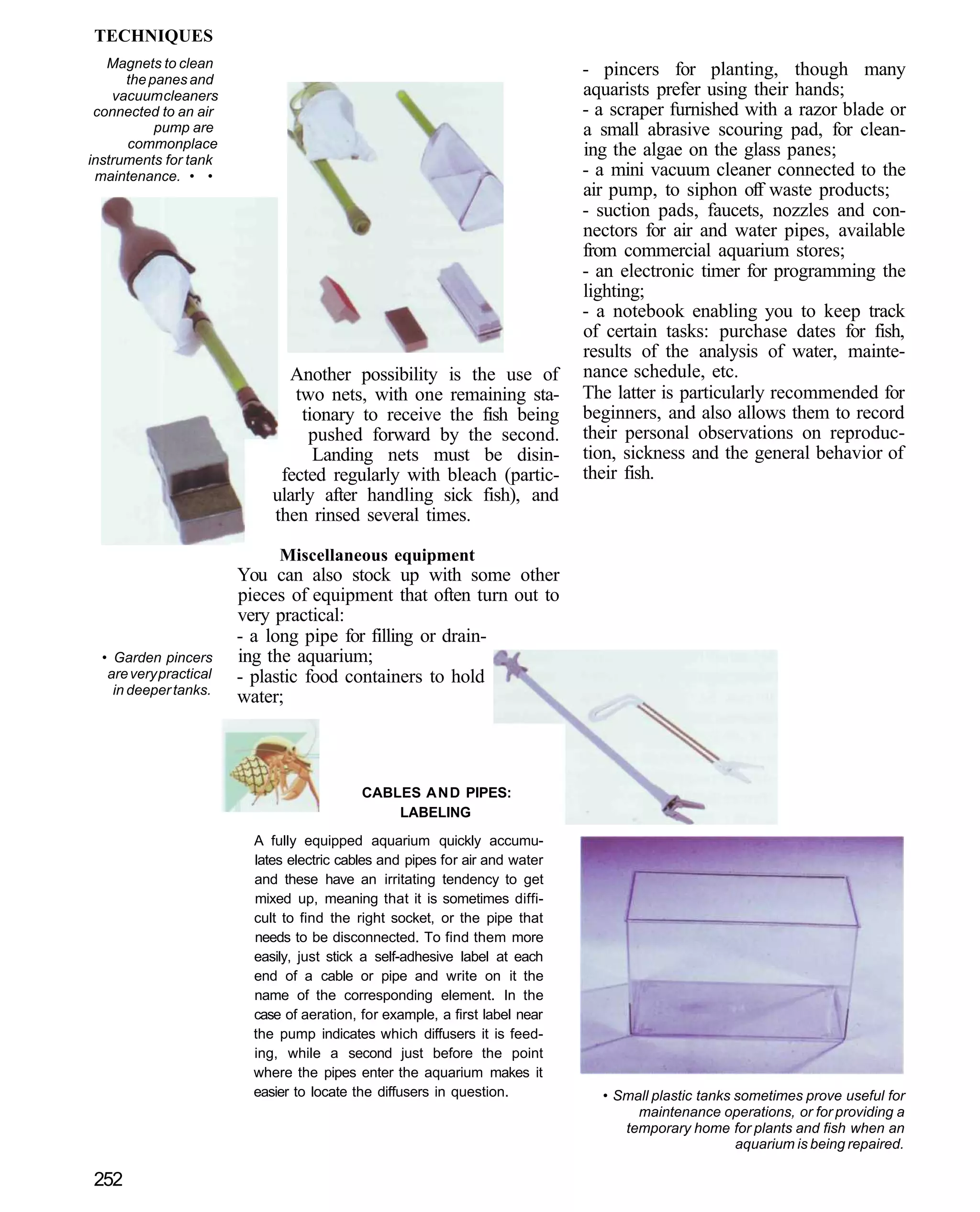
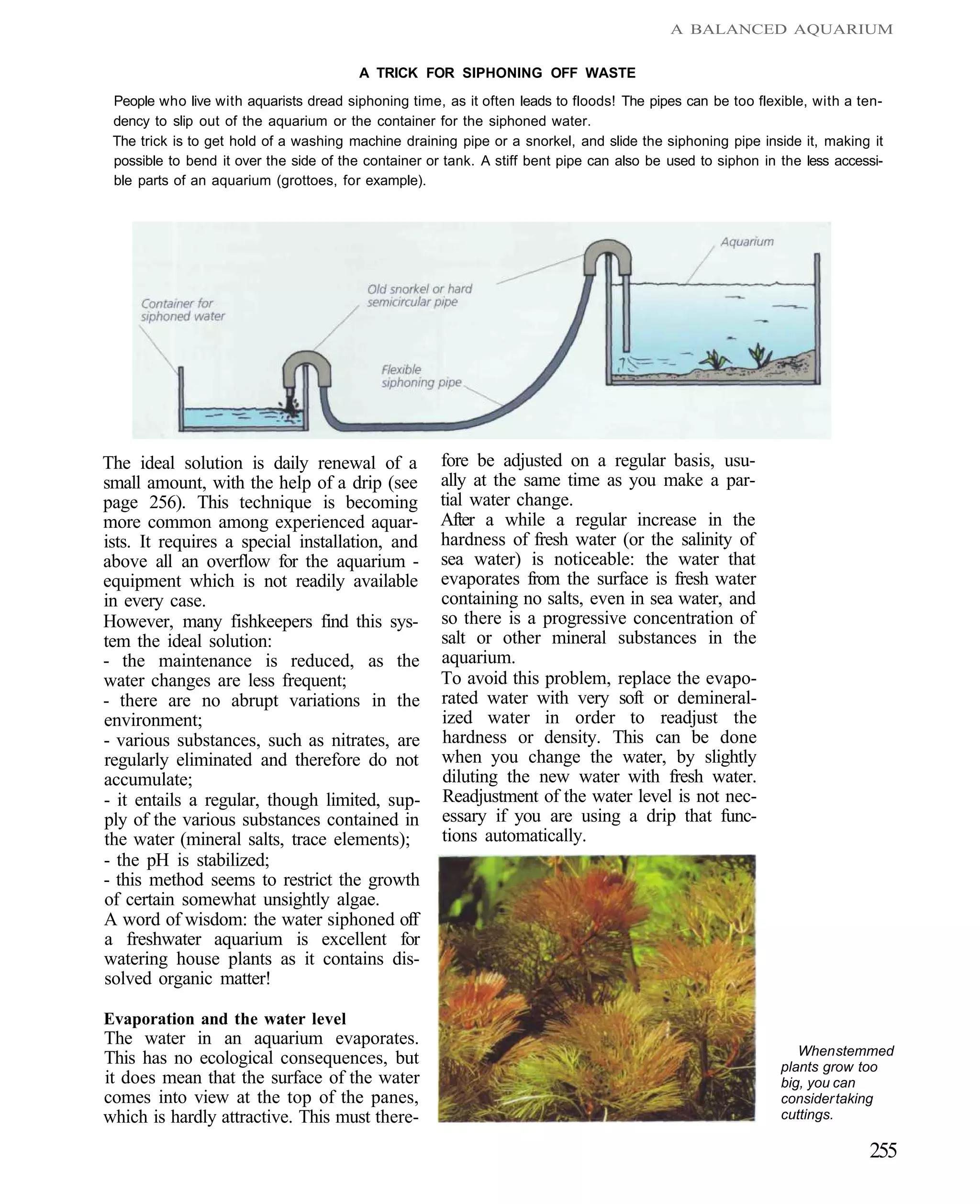
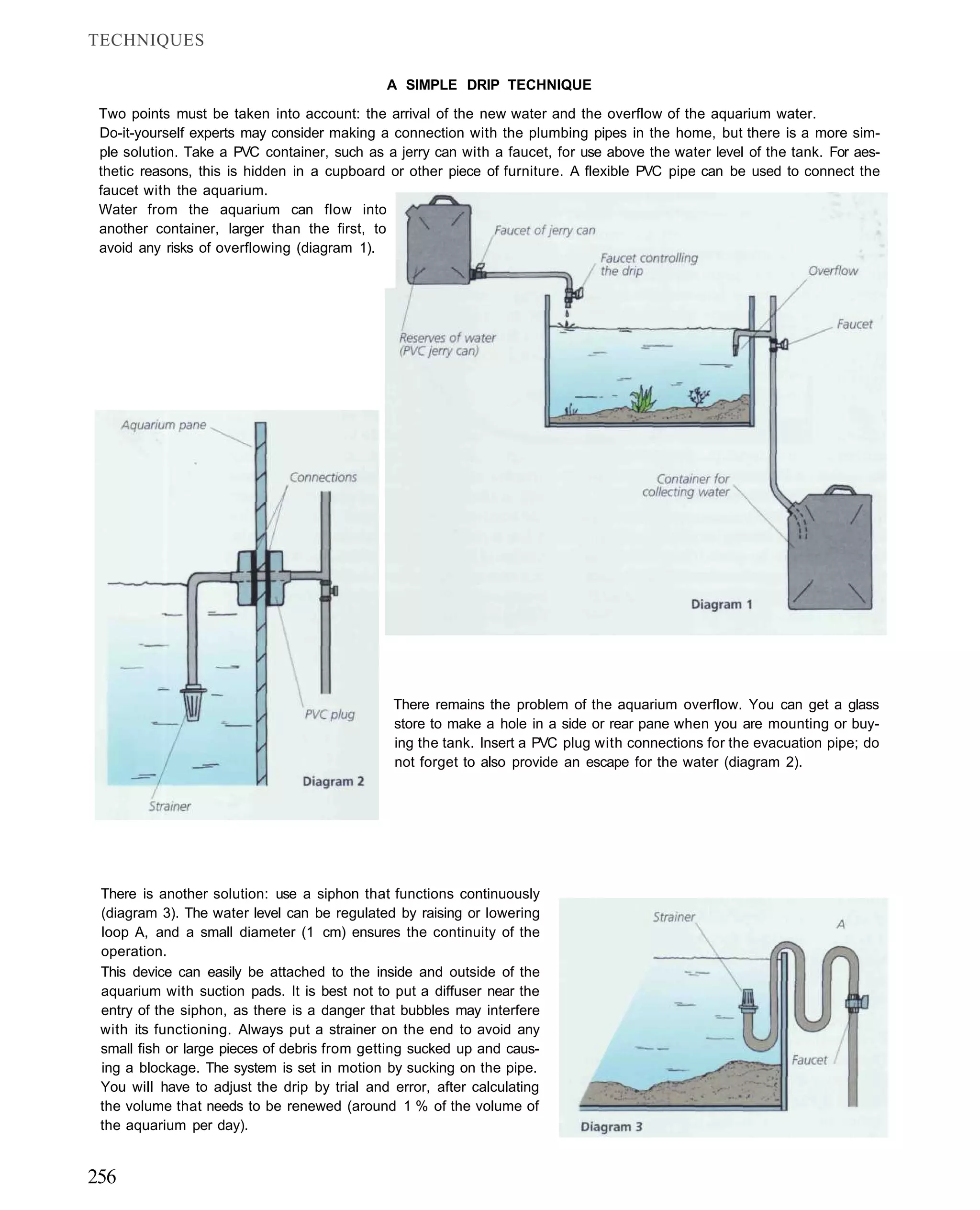
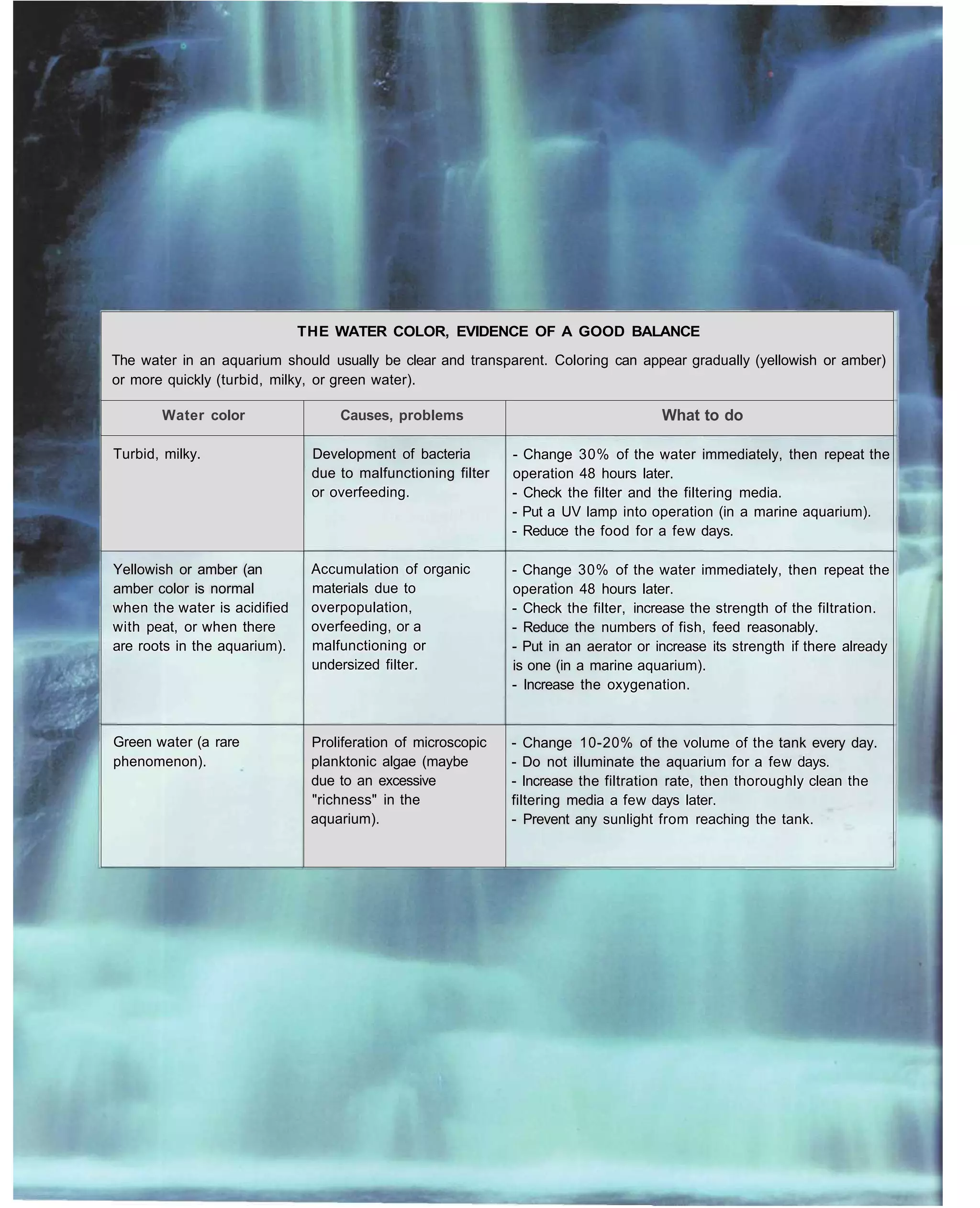
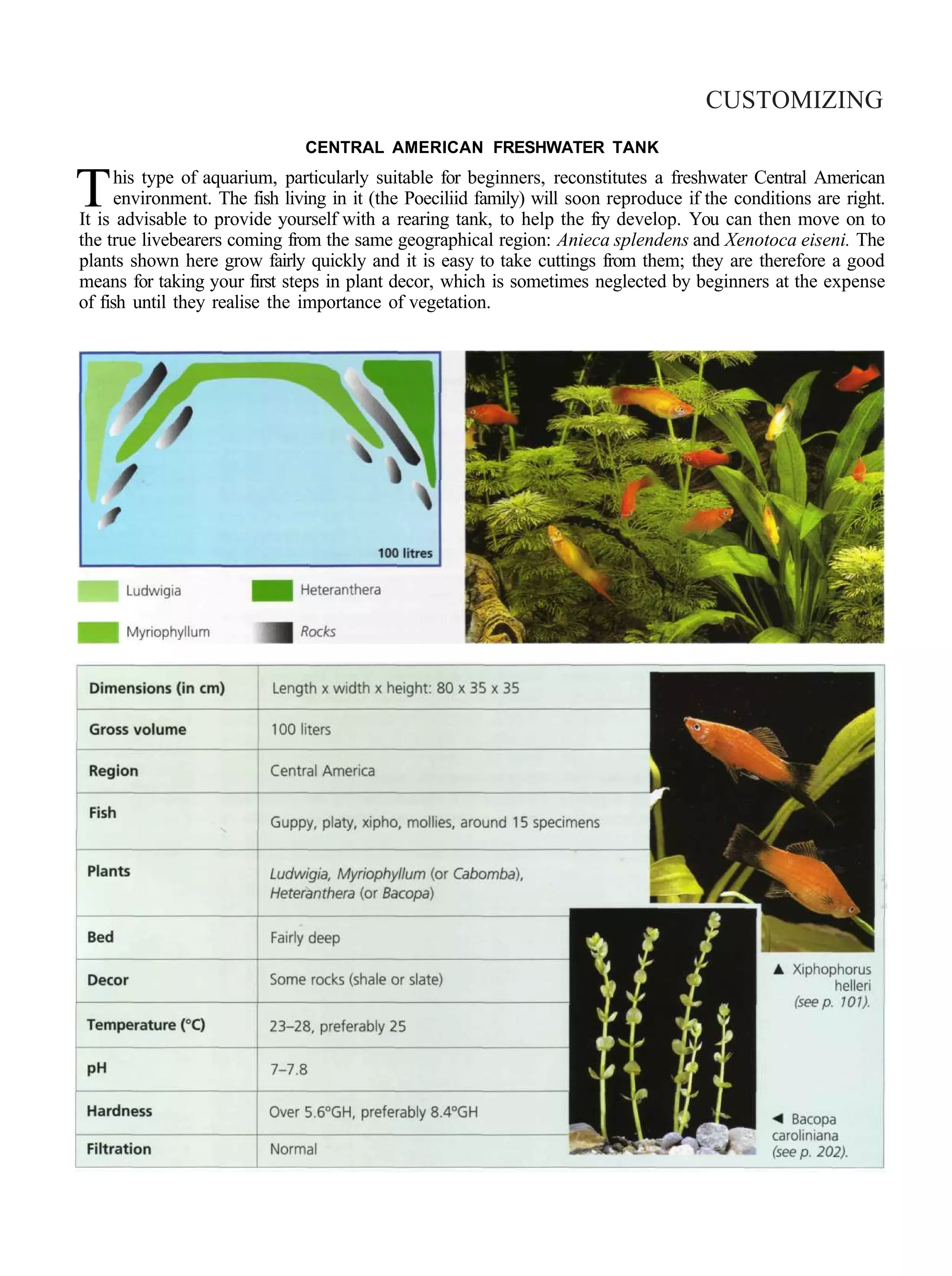
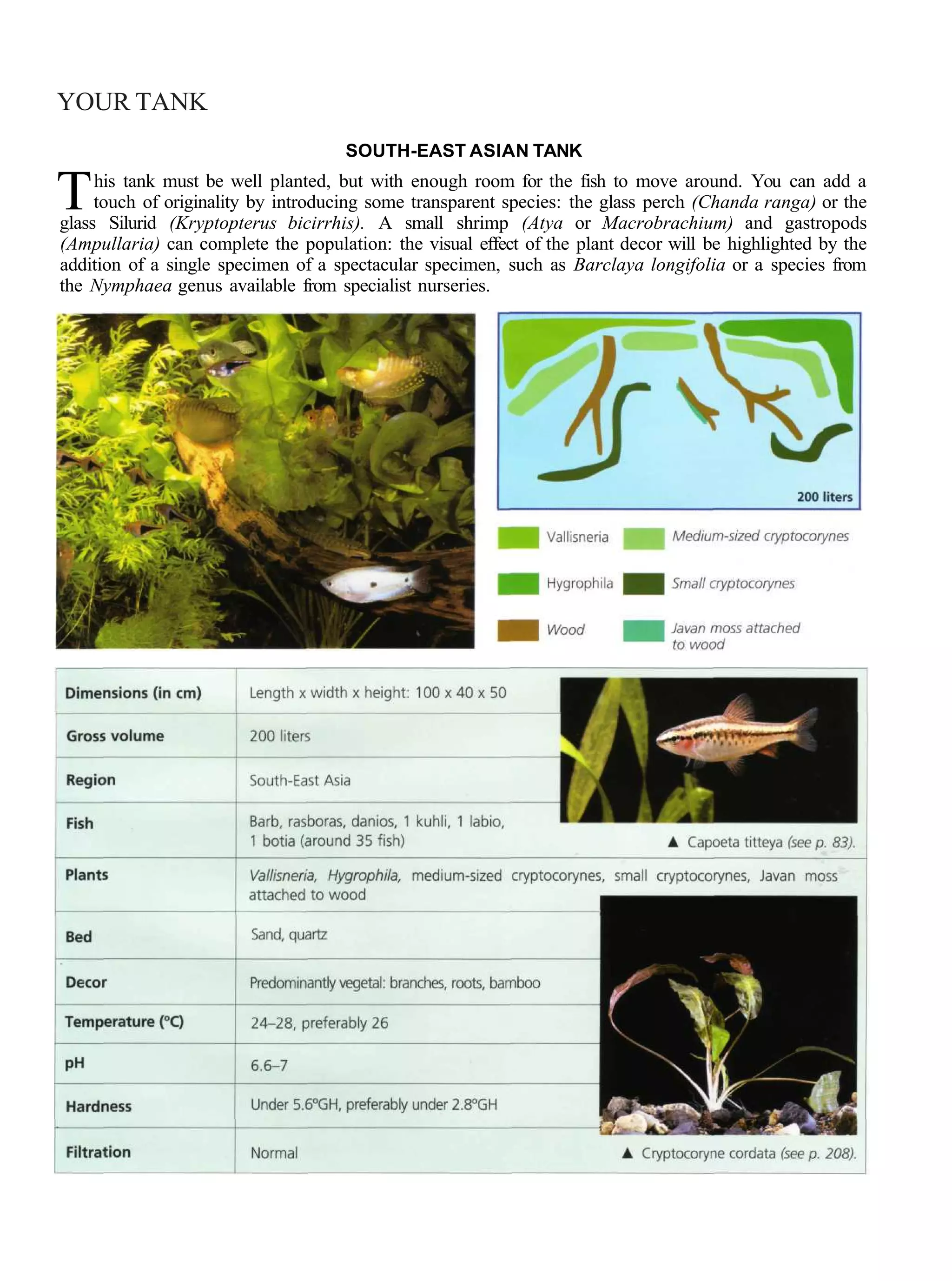
This document provides an overview of key factors for maintaining freshwater aquariums, including:
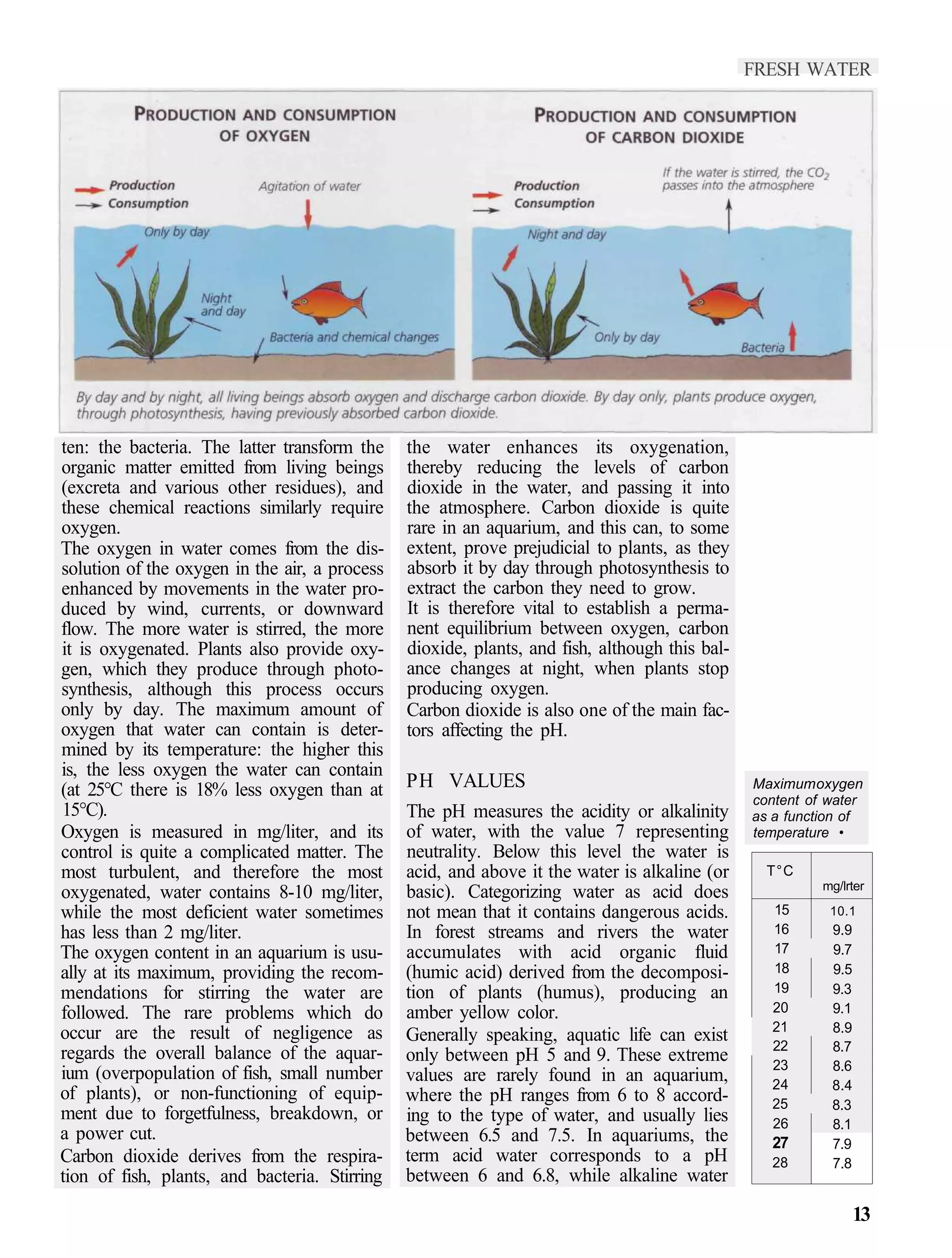
1) Temperature, oxygen, and carbon dioxide levels are important water parameters that must be regulated to support aquatic life. Stirring the water enhances oxygenation.
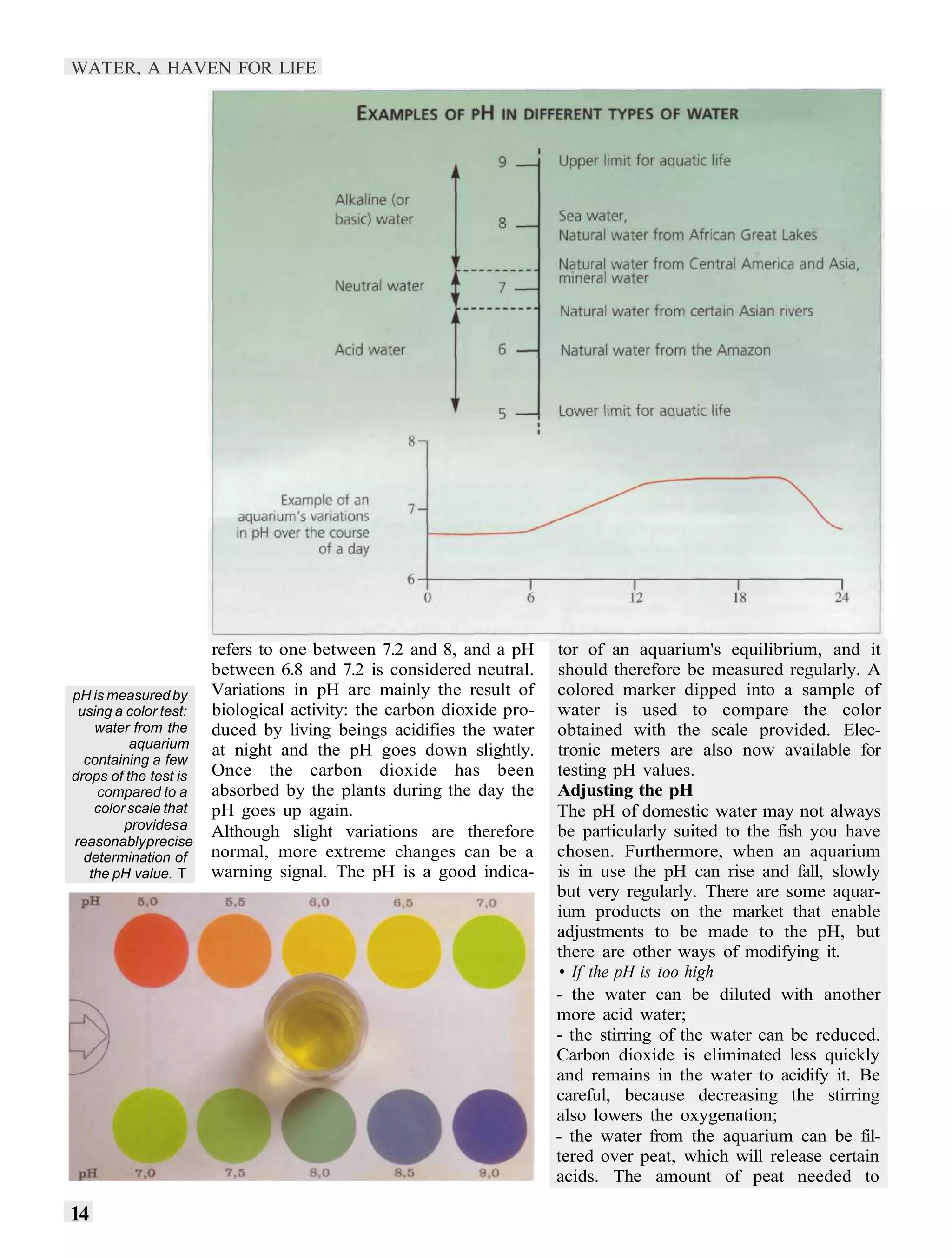
2) pH measures acidity and alkalinity, with most freshwater fish thriving between 6-8. Biological activity from fish, plants, and bacteria can cause the pH to fluctuate.
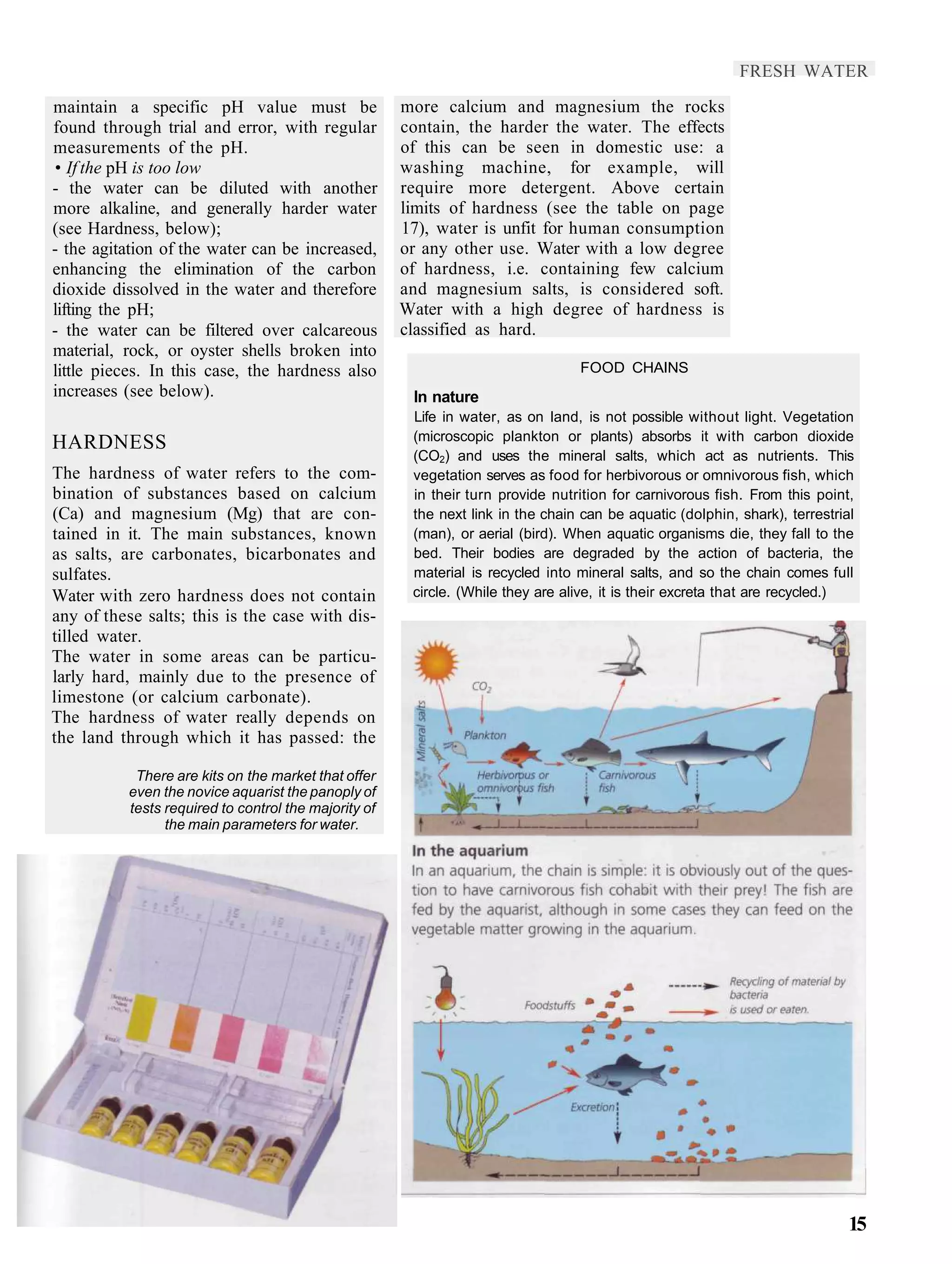
3) Proper balancing of oxygen, carbon dioxide, plants, and fish is needed to maintain equilibrium in the closed aquarium system. Adjustments may be required to suit different fish species.