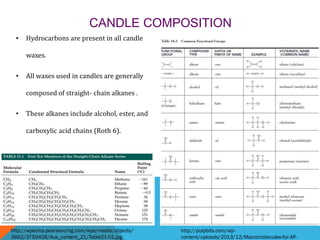
The document discusses the chemistry of candles. It explains that the combustion reaction in candles involves a substance reacting with oxygen to produce carbon dioxide and water. The wick maintains a constant flame to allow this reaction to continue. Most candle wax is made from paraffin, a hydrocarbon. The wick is embedded in the wax to allow it to vaporize slowly and sustain the flame. The flame burns hottest at the top due to absorption of oxygen at the bottom of the flame.