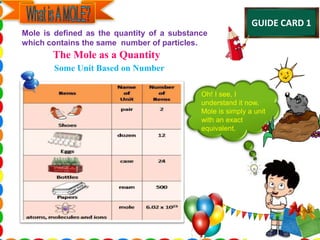
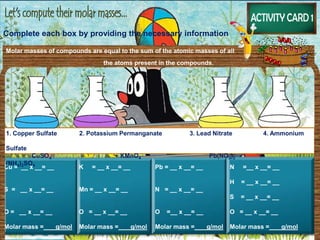
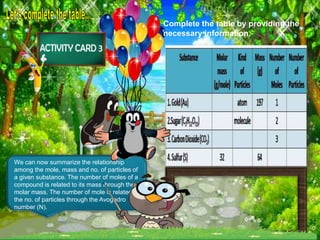
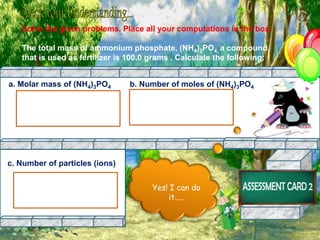
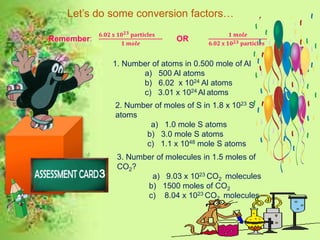
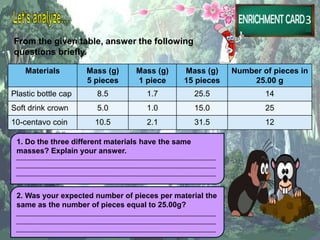
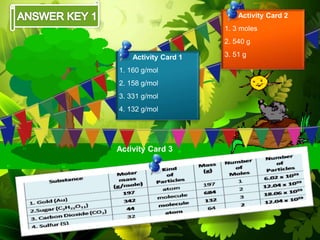
The document discusses the mole concept in chemistry. It provides guide cards explaining what a mole is, how to calculate molar mass, and how to convert between moles, mass, and number of particles. It includes activity cards with practice problems on these topics. The goal is to help students understand intricate topics in chemistry, like the mole, and enhance their critical thinking skills.