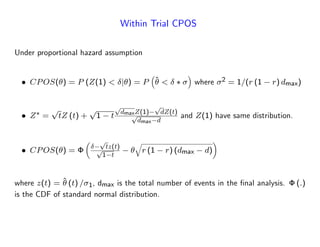
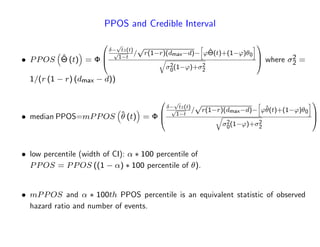
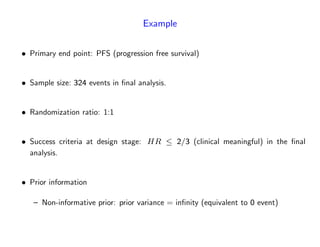
The document discusses a design paradigm for assessing defensive efficacy in clinical trials using the concept of probability of success (POS), emphasizing dynamic decision-making and benefit/risk assessment as mandated by FDA and EMA guidelines. It outlines various types of POS calculations and their applications in interim analysis, detailing the importance of statistical evidence and ethical considerations in drug development. Key takeaways include the adaptability of POS in design modifications and the impact of newly emerged safety signals on efficacy declarations.