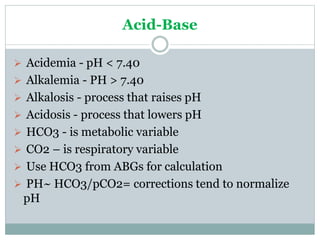
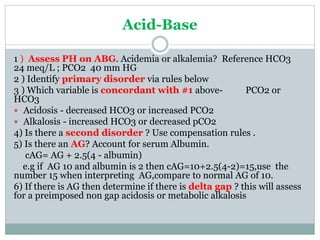
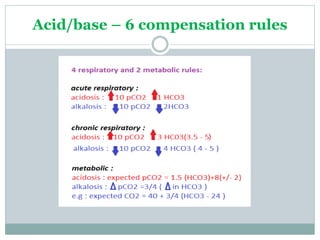
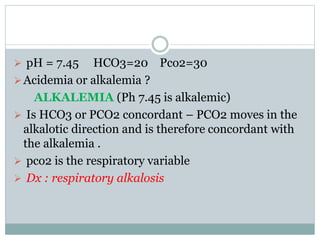
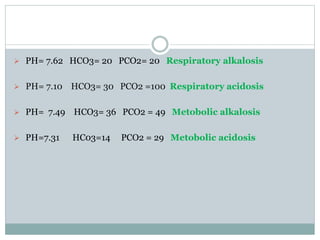
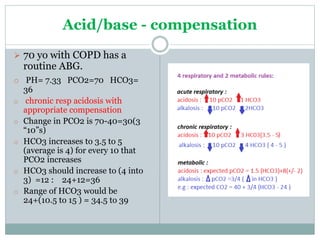
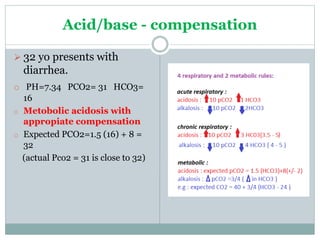
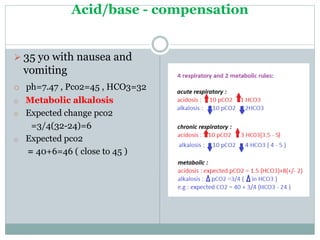
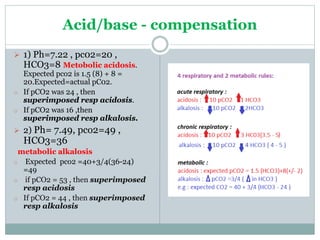
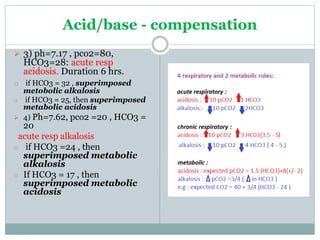
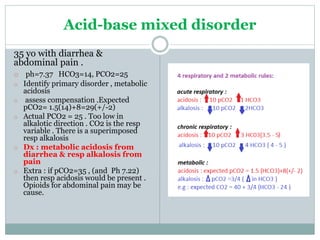
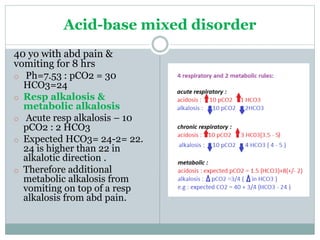
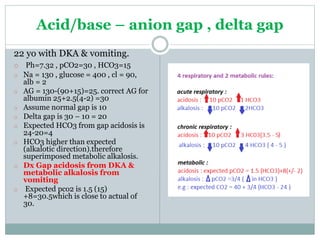
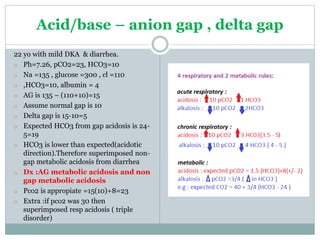
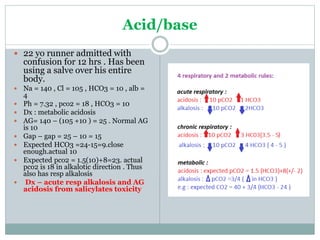
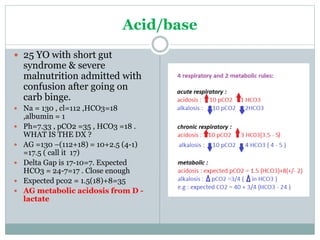
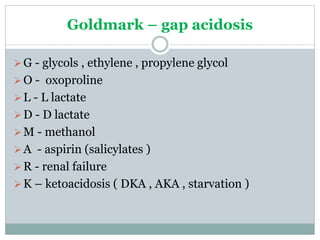
The document discusses the interpretation of acid-base balance in critical care patients, outlining definitions of acidemia, alkalemia, acidosis, and alkalosis while detailing the variables involved (HCO3 and CO2). It provides a step-by-step process for assessing arterial blood gas (ABG) values and identifying primary and secondary disorders, along with compensation rules and examples of various clinical scenarios. Additionally, it covers anion gaps and delta gaps in diagnosing metabolic disorders, emphasizing the importance of patient history in determining underlying causes.