vijayrathodp1.pptx
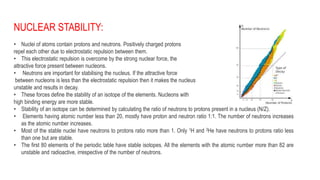
- 1. NUCLEAR STABILITY: • Nuclei of atoms contain protons and neutrons. Positively charged protons repel each other due to electrostatic repulsion between them. • This electrostatic repulsion is overcome by the strong nuclear force, the attractive force present between nucleons. • Neutrons are important for stabilising the nucleus. If the attractive force between nucleons is less than the electrostatic repulsion then it makes the nucleus unstable and results in decay. • These forces define the stability of an isotope of the elements. Nucleons with high binding energy are more stable. • Stability of an isotope can be determined by calculating the ratio of neutrons to protons present in a nucleus (N/Z). • Elements having atomic number less than 20, mostly have proton and neutron ratio 1:1. The number of neutrons increases as the atomic number increases. • Most of the stable nuclei have neutrons to protons ratio more than 1. Only 1H and 3He have neutrons to protons ratio less than one but are stable. • The first 80 elements of the periodic table have stable isotopes. All the elements with the atomic number more than 82 are unstable and radioactive, irrespective of the number of neutrons.
- 2. LAW OF RADIOACTIVE DECAY: When a radioactive material undergoes α, β or γ-decay, the number of nuclei undergoing the decay, per unit time, is proportional to the total number of nuclei in the sample material So, If N = total number of nuclei in the sample and ΔN = number of nuclei that undergo decay in time Δt then, ΔN/ Δt ∝ N Or, ΔN/ Δt = -λN … (1) where λ = radioactive decay constant or disintegration constant. Now, the change in the number of nuclei in the samle,the rate of change of N (in the limit Δt → 0) is, dN/dt = – λN Or, dN/N = – λ dt Now, integrating both the sides of the above equation, we get, N N0∫ dN/N = λ t t0∫ dt … (2) Or, ln N – ln N0 = – λ (t – t0) … (3) Where, N0 is the number of radioactive nuclei in the sample at some arbitrary time t0 and N is the number of radioactive nuclei at any subsequent time t. Next, we set t0 = 0 and rearrange the above equation (3) to get, ln (N/N0) = – λt Or, N(t) = N0e– λt … (4) Equation (4) is the Law of Radioactive Decay.
- 3. HALF-LIFE: Half-Life is normally defined as the time needed by one half of a radioactive substance to disintegrate or transform into a different substance. The principle was first discovered in 1907 by Ernest Rutherford. It is usually represented by the symbol t1/2. N(t) = N0e– λt t1/2 = 0.693/λ t1/2 = half-life λ = decay constant
- 4. MEAN LIFE: in radioactivity, average lifetime of all the nuclei of a particular unstable atomic species. This time interval may be thought of as the sum of the lifetimes of all the individual unstable nuclei in a sample, divided by the total number of unstable nuclei present. Mean lifetime is a very significant quantity that can be measured directly for small number of atoms. If there are ‘n’ active nuclei, (atoms) (of the same type, of course), the mean life is τ = τ1 + τ3 + ... + τ2 / n Where τ1, τ2,....... τn represent the observed lifetime of the individual nuclei and n is a very large number. It can also be calculated as a weighted average: τ = τ1N1 + τ3N2 + ... + τ2Nn / N1 + ... + Nn Where N1 nuclei live for time τ1, N2 nuclei live for time τ2....... and so on. Using calculus we may rewrite it as: τ = 0 ∞ 𝑡𝑑𝑁 0 ∞ 𝑑𝑁 Where |dN| is the number of nuclei decaying between t, t + dt; the modulus sign is required to ensure that it is positive. dN = –λN0e–λt dt and |dN| = λN0e–λtdt
- 5. or τ = 1/λ. The half-life and the mean life of substances are related to each other by the formula: T = τ ln 2 ≈ 0.693 τ The two parameters vary drastically for different substances. For example the half-life of Polonium-212 is less than 1 microseconds, while for Thorium-232, the half-life crosses even 1 billion years.